Fig. 20.1
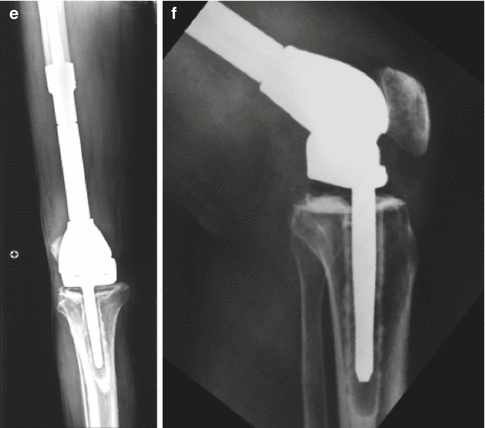
A 70 years old male presented with progressive knee- distal femur pain over 3 months. (a) X-ray reveals a predominantly lytic lesion with intra-lesional calcifications of the distal metaphysis- diaphysis of the femur. (b) MRI axial T2 FS image reveals cortex erosion and soft tissue extension. Closed biopsy was consistent with high grade chondrosarcoma. (c) The patient underwent wide tumor resection. Distal femur specimen. (d) Cemented rotating hinge prosthesis was inserted. (e, f) X-rays 3 months post operatively
Cemented Fixation
Unwin et al. in 1996, reviewed 1,001 Stanmore cemented custom made prostheses with fixed hinge mechanisms, inserted before 1992 as a primary replacement for bone tumor [9]. The probability of avoiding aseptic loosening for 10 years was reported as 93.8 % for the proximal femur, 67.4 % for the distal femur and 58 % for proximal tibia replacements. The amputation rate due to complications for the entire group was 8.6 %. Myers et al. in 2007, reported on 335 patients who underwent distal femoral replacement [24]. A total of 192 patients remained alive with a mean follow-up of 12 years. All prostheses were custom made. One hundred and sixty two patients had a fixed-hinge design and 173 a rotating-hinge of which 143 had a hydroxyapatite (HA) collar. Only 15 prostheses were uncemented. Patellar resurfacing was not routinely performed. Early failure was usually due to infection or breakage of the prosthesis whereas late failure was more likely to be due to aseptic loosening. If aseptic loosening was taken as the endpoint, the rotating-hinge design with an HA collar was least likely to fail. The risk of revision for aseptic loosening of a fixed-hinge was 35 % at 10 years compared with 24 % for a rotating-hinge without an HA collar and 0 % for a rotating-hinge with an HA collar. Rebushing of the primary endoprosthesis was needed in 55 prostheses (45 fixed-hinge, 10 rotating-hinge). The overall infection rate was 9.6 %. Amputation was performed in 6 % for local recurrence and in 4.5 % of patients due to infection. Schwartz et al. in 2009, compared 85 modular distal femoral implants to 101 custom-casted designs [12]. All prostheses were cemented with a rotating hinge mechanism. The modular components had a greater 15-year survivorship than the custom-designed implants: 93.7 % versus 51.7 %, respectively. 9.7 % of the patients ultimately required amputation. The authors conclude that long-term survivors should expect at least one or more revision procedures in their lifetime. Bergin et al. in 2012, published the results of 104 distal femoral reconstructions [25]. They focused their analysis on the impact of the bone/stem ratio on aseptic loosening rate. All patients received a cemented modular prosthesis. Survival for 104 stems from aseptic loosening was 94.6 % at 10 and 15 years and 86.5 % at 20 years. The bone/stem ratio independently predicted aseptic failure. Patients with stable implants had larger stem sizes and lower bone/stem ratios than those with loose implants (14.5 mm versus 10.7 mm and 2.02 versus 2.81, respectively). The largest cause of failure in this study was infection (11.7 %) while 5.8 % of the implants were revised because of stem fracture.
Uncemented Fixation
Batta et al. has reported a high rate of aseptic loosening for custom-made uncemented, distal femoral endoprosthetic replacements [26]. Nine out of 69 implants (13 %) had to be revised due to aseptic loosening. All aseptically loose implants were diagnosed within the first 5 years. Capanna et al. in 1993, reports the results of 95 modular uncemented KMFTR tumor prostheses for distal femoral resections [27]. The femoral stem had two lateral flanges at right angles to each other, each with three holes to allow the passage of a total of six screws through the stem and cortex. Clinical results were excellent or good in 75 %. Local recurrence of the tumors developed in five patients. The polyethylene bushes failed in 42 % of cases at an average of 64 months postoperatively, causing varus-valgus instability or locking, usually painless. The infection rate was 5 % for primary cases and was correlated to the extent of quadriceps excision. Bone remodeling around the femoral stem was evaluated on X-rays using the Rizzoli system. According to this system, in grade A there is no change, Grade B there is cortical sclerosis, Grade C there is cortical cancellation, Grade D there is distal sclerosis and proximal atrophy and in Grade E there is proximal osteolysis. In their series Grade D remodeling occurred in 47 % of the prostheses fixed with six screws and in only 11 % with three screws. Stem breakage occurred in 6 % and was associated with the use of narrow stems and extensive quadriceps excision. Most of the fractures occurred though the proximal screw hole. Lan et al. used dual-energy X-ray absorptiometry (DEXA) to evaluate the extent of periprosthetic bone remodelling around the KMFTR prosthesis for distal femoral reconstruction [28]. Bone loss around the KMFTR prosthesis was maximal at the distal end of the femur and progressively decreased towards the proximal end of the stem. Ten patients with implants fixed by screws were found to have a mean loss of bone mineral density (BMD) of 42 % in the most distal part of the femur, while the 13 without screw fixation had a mean loss of 11 %. Mittermayer et al. in 2002, reported on 251 uncemented reconstructions with the KMFTR system or the Howmedica Modular Reconstruction System (HMRS) [29]. Aseptic loosening rate at 10 years was 4 % for proximal femur, 24 % for distal femur replacements and 15 % for proximal tibia. The first radiological signs of aseptic loosening were always seen at the most proximal or distal part of the anchorage stem at a mean of 12 months after the first implantation. Griffin et al. in 2005, examined the risk factors associated with prosthetic failure for the KMFTR uncemented tumor prosthesis of 74 distal femoral [30]. For the distal femoral prosthesis the aseptic loosening rate was very low (2.7 %), the infection rate was 6.8 %, tumor local recurrence was 6.8 %, and the stem fracture rate 5.4 %. All stem fractures occurred through components with six holes for transverse screw fixation produced before 1994. No fractures occurred through newer components with only three holes.
Proximal Tibia Replacements
For proximal tibia resections the common surgical approach is the anterior with proximal medial femoral extension, allowing for popliteal space exploration, identification of the popliteal neurovascular bundle, the trifurcation of the popliteal artery, arterial branches to gastrocnemius heads and common peroneal nerve (Fig. 20.2).
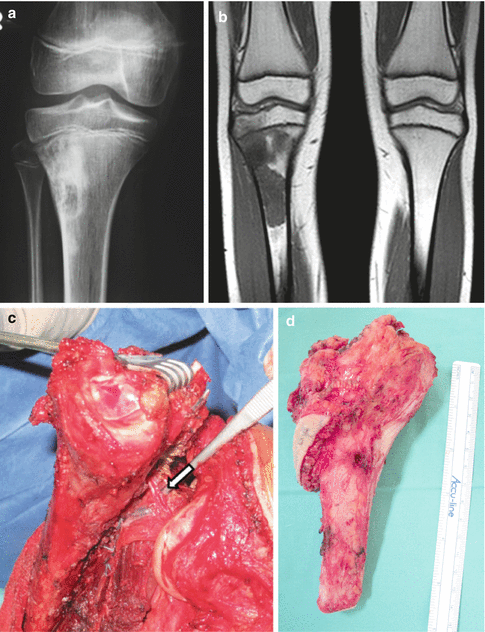
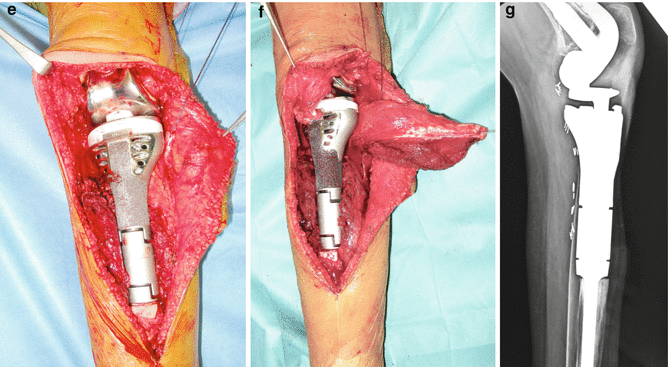


Fig. 20.2
A 12 years old female had right vague proximal tibia pain for a month. (a) anterior posterior x-ray reveals mixed sclerotic and lytic areas at the metaphyseal area. (b) MRI T1 coronal image shows a low to iso-intense signal to muscle lesion of the metaphysis extending to proximal tibial epiphysis. Biopsy of the lesion diagnosed osteosarcoma. The patient followed neo-adjuvant chemotherapy (c) Intraoperative view. Popliteal artery is dissected and the branch of the anterior tibial artery (arrow) is identified and ligated. (d) The proximal 14 cm of the tibia with proximal fibula 5 cm was resected en block. (e) A cemented modular hinge rotating prosthesis is inserted 1 cm longer. (f) The patellar tendon is sutured with heavy sutures over the porous coated anterior surface of the prosthesis. The medial gastrocnemius head is dissected and ready to rotate over the prosthesis and tendon attachment. (g) Lateral x-ray 6 months post operatively. The patient had 10° extension lag
The reported results for proximal tibia replacement megaprostheses are frequently inferior to the distal femur. Two inherent characteristics of proximal tibia resection surgery are considered to be the principal causes for this outcome: defective attachment of the patellar tendon and the lack of available soft tissue. The attachment of the patellar tendon should be resected at least a few millimeters from the tibial tubercle in cases of malignancy, in order to achieve a clear oncological margin, thus resulting in a shortened tendon stump. In order to restore the continuity of the extensor mechanism, augmentation of the stump with synthetic or biological material is frequently necessary. This is the weak point for this step of reconstruction as reliable and effective long-term attachment of the tendon to the implanted prosthesis is not always successful. We frequently observe a gradual proximal migration of the patella on a lateral X-ray and a clinical lag of active, but full passive knee extension. Colangeli et al. in 2007, performed gait analysis of knee megaprostheses for proximal tibia tumors [31]. Functional performance during gait was abnormal in moss cases, consistent with weakness of the extensor apparatus and knee extension lag. Knee stability was supported by the intrinsic prosthesis biomechanics. The inadequacy of surrounding soft tissue for tension free coverage of the prosthesis (especially the metaphyseal part of the prosthesis) is the other major issue. Frequently, the proximal tibia has to be resected en block with the proximal fibula and an envelope of soft tissue for safe negative tumor margins. An important step in the evolution of limb sparing surgery for proximal tibia tumors was the concept of a local rotational flap of gastrocnemius head for prostheses coverage and anchorage of the patellar tendon [32, 33]. More recently the use of the Trevira attachment tube has been introduced to enhance joint capsule stability and tendon attachment [34]. The tube is directly attached to the tibial prosthesis with non-absorbable sutures. Fibroblasts migrate into the tube’s mesh, so that attachment of soft tissue takes place. In Hardes et al.’s series most of the patients were able to actively extend their knee [35].
Cemented Fixation
Myers et al. in 2007, reported on 194 patients who underwent a cemented proximal tibial replacement, with 95 having a fixed hinge design and 99 a rotating-hinge with a hydroxyapatite collar [10]. The median age of the patients was 21.5 years. At a mean follow-up of 14.7 years, 115 patients remained alive. Rebushing of the primary endoprosthesis was needed in 36 patients (20 fixed-hinge, 16 rotating-hinge). The risk of revision for aseptic loosening in the fixed-hinge knees was 46 % at 10 years. This was reduced to 3 % in the rotating-hinge knee with a hydroxyapatite (HA) collar. Amputations were carried out in 17.5 % of patients either for local tumor recurrence or infection. Before gastrocnemius flaps were used the risk of amputation at 10 years following surgery was 28 %. Since the introduction of flaps, this has fallen to 14 %. Schawrtz et al. in 2010, retrospectively reviewed 52 cemented proximal tibial endoprosthetic reconstructions [12]. All prostheses had rotating hinge mechanisms; in 98 % this was the Kinematic rotating-hinge mechanism. Post-operatively all patients had their knee immobilized for a month. The failure of the rotating-hinge mechanism necessitating replacement of the bushings, axle, tibial bearing, or polyethylene was 23.1 % at a mean of 8.9 years postoperatively. Delayed wound healing or minor postoperative wound dehiscence was observed in 13.5 % of patients. The incidence of deep infection and local recurrence rate was 5.8 % and 5.8 % respectively while amputation had to be performed in 9.6 % of the patients. The use of an extramedullary porous ingrowth surface was associated with a lower incidence of aseptic loosening [12, 36]. The 29 modular implants demonstrated a trend toward improved survival compared to the 23 custom-designed components, with a 15-year survivorship of 88 % versus 63 %. The final mean postoperative Musculoskeletal Tumor Society score at was 82 % of normal function [12].
Uncemented Fixation
Griffin et al. in 2005, examined the risk factors associated with prosthetic failure for the KMFTR uncemented tumor prosthesis in 25 proximal tibial implants [30]. For the proximal tibia prosthesis the aseptic loosening rate was 0 %, the infection rate 20 % and stem fracture rate 8 %. Flint et al. in 2006, reported on 44 uncemented proximal tibia reconstructions [37]. Although they had no case with aseptic loosening, 24 % of the prosthesis failed either due to infection, local tumor recurrence, stem fracture, rotational instability or vascular compromise. In 16 % of the patients amputation was carried out. The mean knee extension lag was 6° and the MSTS score was 75 %. Mavrogenis et al. in 2013, reviewed 225 patients with proximal tibial tumors treated with proximal tibial resection from 1985 to 2010 [38]. The prostheses used in this series were KMFTR, HMRS and the rotating hinge Global Modular Reconstruction System (GMRS). Fixation of the prosthesis was cementless in 209 and cemented in 16 patients. The overall survival of patients with sarcomas was 62 % at 10 years, while survival of megaprosthetic reconstructions was 78 % at 10 years, without any difference between fixed and rotating hinge megaprostheses. The overall complication rate was 25 %. The most common complications were infection (12 %), aseptic loosening (6 %), and extensor mechanism rupture (3 %). Infection rate was almost double in patients who had been administered chemotherapy. The mean extension lag from full active extension was 12°. MSTS function was significantly better in multivariate analysis for rotating compared to fixed hinge megaprostheses.
Infection
Infection is a frequent complication of knee megaprosthesis reconstruction ranging from 3.6 % to 37.5 %, and it is a leading cause for amputation [12, 39–44] (Fig. 20.3). Body image is significantly worse for patients undergoing late amputation after failed limb salvage [45]. Hardes et al. in 2006, reported on 30 patients with an infection associated with a tumor endoprosthesis [46]. Limb salvage related to the complication infection was achieved in 63.3 %. The mean number of revision operations per patient was 2.6. No patient receiving chemotherapy with a poor soft tissue condition had limb salvage surgery. A poor soft tissue condition was a significant risk factor for failed limb salvage. Jeys and Grimer, in 2009, stated that the risk of infection is life-long although infection most frequently occurs within 12 months from the last surgical procedure [43]. The most common pathogenic organism is coagulase-negative Staphylococcus and the most effective treatment for deep infection is two-stage revision [43, 47]. Previous radiotherapy increases the infection rate [47]. Flint et al. in 2007, reported on 11 patients who underwent removal of the prosthesis for infection [48]. They concluded that two-stage revision of uncemented tumor endoprostheses with retention of a well-ingrown stem could be associated with successful eradication of infection. Racano et al. in 2013, conducted a systematic review of the literature for clinical studies that reported infection rates in adults with primary bony malignancies of the lower extremity treated with surgery and endoprosthetic reconstruction [49]. This review yielded 48 studies reporting on a total of 4,838 patients. The overall pooled weighted infection rate for lower-extremity LSS with endoprosthetic reconstruction was approximately 10 % with the most common causative organism reported to be Gram-positive bacteria in the majority of cases. The pooled weighted infection rate was 13 % after short-term postoperative antibiotics and 8 % after long-term postoperative antibiotics. Silver is well known for its anti-microbial properties. Silver coated megaprostheses are currently under investigation regarding their effect on incidence of deep infection and possible side effects [50, 51]. An in-vivo study in a rabbit model concludes that the silver coated Mutars megaprosthesis resulted in reduced infection rates without toxicological side effects [52]. Hardes et al. demonstrated a lower infection rate and less aggressive treatment of infection in patients treated with silver coated megaprostheses compared to titanium prostheses [53]. Shirai et al. in 2014, performed a clinical trial of iodine-coated megaprostheses to evaluate their safety and antibacterial effect [54]. Abnormalities of thyroid gland function were not detected. The authors conclude that the iodine-supported titanium megaprostheses were highly effective and showed promise in the prevention and treatment of infections in large bone defects.