Chapter 78 Citicoline (CDP-Choline)
 Introduction
Introduction
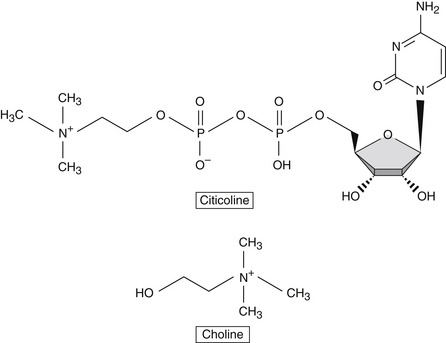
Citicoline is also an exogenous source for acetylcholine synthesis, a key neurotransmitter, and a member of the group of molecules that play important roles in cellular metabolism known as nucleotides.1
First identified in 1955 by Kennedy et al,2 and synthesized in 1956, citicoline has been studied in Europe, Japan, and the United States for several decades. It is widely available as an approved drug for the treatment of neurologic disorders in many countries and is sold as a dietary supplement in the United States.
Citicoline Versus Choline
Choline is a component of the diet and is produced in the brain, albeit in small amounts. Due to its low endogenous production, it is considered an essential nutrient and classified with the B vitamin complex. It plays several essential roles in human physiology, including enhancement of structural integrity and signaling for cell membranes, supporting acetylcholine synthesis, and synthesis of betaine, a methyl donor.3
When taken orally, citicoline is hydrolyzed in the intestinal tract and in circulation to form choline and cytidine, which is the nucleoside of cytosine. Citicoline provides the brain with a source of choline and cytidine, which are efficiently used in the Kennedy cycle to generate phospholipids. Although choline on its own is preferentially used for the synthesis of acetylcholine, cytidine is highly efficiently used in the brain for the synthesis of various nucleotides (Figure 78-1). Studies in neuronal cell lines showed that cytidine administration increased the incorporation of choline into membrane phosphatidylcholine.4
In terms of safety, choline is a substance with a low level of toxicologic concern. Administering choline with cytidine, in the form of citicoline, lowers the toxicity index by twenty-fold.5 Furthermore, citicoline administration is significantly different than administration of choline in cases of cerebral ischemia caused by stroke and other conditions. Citicoline’s therapeutic effects in such conditions stem from its ability to: (1) increase phosphatidylcholine synthesis, the primary component of neuronal membranes; (2) enhance acetylcholine synthesis, ameliorating the symptoms resulting from ischemic loss of cholinergic neurons; (3) promote the synthesis of several other membrane phospholipids, including phosphatidylethanolamine and phosphatidylserine, leading to repair and regeneration of axons and synapses; and (4) prevent the accumulation of free fatty acids and the generation of free radicals at the site of ischemia, thereby preventing the initiation of a proinflammatory cascade of events.5
 Bioavailability/Pharmacokinetics
Bioavailability/Pharmacokinetics
The pharmacokinetics of an oral dose of 14C-labeled citicoline has been studied in humans. Administration of a single 300 mg dose to healthy adults was shown to have nearly complete absorption, with less than 1% of the labeled compound found in feces after a 5-day collection period. Absorption of citicoline gave rise to two chromatographic peaks in concentrations of radioactivity in plasma, the first at 1 hour, and the second, larger peak at 24 hours after dosing. The main route of excretion was found to be via respiratory carbon dioxide, with significant excretion also occurring through urine (Figure 78-2). After 5 days, 16% of the administered dose was recovered, suggesting that the remainder was incorporated into tissues or was available for biosynthetic and biodegradative pathways.6

FIGURE 78-2 Major metabolic pathways for citicoline.
Modified from Weiss GB. Metabolism and actions of CDP-choline as an endogenous compound and administered exogenously as citicoline. Life Sci. 1995;56(9):637-60.
A pharmacokinetic study in rats using 14C-methyl-labeled citicoline confirmed almost complete absorption with oral administration, with calculated oral bioavailability being approximately 92% of that obtained from intravenous (IV) dosing. The absorption was categorized as slow and complete with sustained blood levels, the highest being at around 5.5 hours after administration. Radioactive labeling found citicoline and its metabolites widely distributed throughout tissues, including distribution of metabolites to the brain, confirming their ability to participate in the synthesis of phospholipids.7
A confirmatory study, again using radio labeled citicoline in rats, found 62.8% of total radioactivity was distributed in brain tissue as phospholipids, including phosphatidylcholine and sphingomyelin. These results suggested that metabolites of orally administered citicoline were available in the brain for resynthesis of endogenous citicoline.8 Although only a small percentage of the total citicoline dose crosses the blood–brain barrier as choline and cytidine, the utilization of these precursors in brain tissue for phospholipid biosynthesis is extremely efficient.4
 Mechanism of Action
Mechanism of Action
Citicoline has several important mechanisms of action, leading to a broad range of beneficial effects on neurologic function. In cerebral ischemia, citicoline primarily acts by increasing the synthesis of phosphatidylcholine, the primary neuronal membrane phospholipid, and enhancing the production of acetylcholine. Oral citicoline administration increases plasma levels of choline and cytidine, which are building blocks used to restore neuronal membrane integrity.5
Interestingly, citicoline seems to have differential effects on phosphatidylcholine synthesis in younger versus older adults. Phosphatidylcholine is an essential compound for cell membrane integrity and repair. It is normally reduced in brain cell membranes as a result of aging. A study using protein magnetic resonance spectroscopy to measure brain concentrations of cytosolic choline-containing compounds before and after a single oral dose of citicoline found that the choline resonance in the brain of younger individuals increased, whereas it decreased in older subjects. It was presumed that the cytidine component of citicoline enhanced the incorporation of brain choline into neural cell membrane phosphatidylcholine in older subjects, resulting in the decrease.9
The ability of citicoline to stimulate brain phospholipid synthesis in humans was further supported by studies showing that healthy subjects consuming 500 mg/day orally for 6 weeks (administered as Cognizin citicoline) had increased levels of phosphodiesters in brain tissue, such as glycerophosphocholine and glycerophosphoethanolamine, as assessed by phosphorus magnetic resonance spectroscopy. These findings supported citicoline’s ability to increase phosphatidylcholine synthesis.10 Findings from a study of healthy middle-aged adults confirmed these results, but suggested that the increase in phosphorus metabolites attributed to citicoline intake was regionally specific, with the frontal lobe being the preferred site of deposition, ultimately enhancing frontal lobe energetics and improving phospholipid membrane turnover. This area of the brain contributes to memory function by supporting vigilance, attention, and working memory capacity, and by reducing mental fatigue. Because citicoline’s effect was most prominent in this brain region, this is a likely explanation for its clinical benefit of improved cognitive function.11
Citicoline may further benefit patients experiencing ischemia by decreasing the accumulation of free fatty acids at the site of the lesion, which occurs as a result of neuronal cell damage and death. Soon after initiation of ischemia, there is a significant increase in proinflammatory arachidonic acid, glycerols, and free fatty acids, caused by the breakdown of neuronal membranes. Toxic metabolites as well as prostaglandins, thromboxanes, and free radicals can accumulate, leading to further damage. Animal studies showed that intracerebral administration of citicoline before induction of ischemia reduced the increase in free fatty acids, arachidonic acid, and other toxic metabolites, attenuating free radical damage and restoring membrane function.5
Some evidence points to the ability of citicoline to normalize neurotransmitter release patterns. In conditions of cerebral hypoxia, which exist in ischemia, norepinephrine release may decrease, whereas the release of dopamine may increase. In several animal models, citicoline was shown to inhibit the impairment of neurotransmitter release in hypoxic conditions. Furthermore, citicoline administration to rats kept in a chronic hypoxic state reduced behavioral deteriorations and increased survival time. Additional studies found that citicoline was able to increase the dilation of blood vessels in animals with cerebral microcirculation injury, significantly increasing cerebral blood flow.4
Citicoline shows neural restorative effects, presumably via action on the dopaminergic system of the central nervous system. Rats with substantia nigra lesions were shown to regenerate nerve cells after treatment with citicoline, indicating its protective effect in this region. Further studies found that citicoline administration to rats increased striatal dopamine synthesis. Several other investigations in animal models yielded evidence of citicoline’s ability to enhance dopaminergic synthetic pathways.4 This was a result of the activation of tyrosine hydroxylase and inhibition of dopamine reuptake, which is related to citicoline’s activity on phospholipid synthetic pathways. Citicoline is also known to have effects on serotonin and norepinephrine.12 Studies in rats showed that citicoline improved learning and memory capacity and enhanced motor performance and coordination in aged rats. These findings provided further evidence for citicoline’s cholinergic activity.13
Additional mechanisms of citicoline’s neuroprotective effects were highlighted in recent research. Studies suggested that citicoline enhanced the preservation of an inner mitochondrial membrane component known as cardiolipin, which is important for preservation of mitochondrial function. Citicoline facilitated the preservation of sphingomyelin, which promotes signal transduction in nerve cells. Citicoline exhibited direct antioxidant effects, because research showed that it has an ability to stimulate glutathione synthesis and activity of the enzyme glutathione reductase. Furthermore, citicoline attenuates lipid peroxidation. These downstream effects may be attributable to citicoline’s larger function of attenuating the activation of phospholipase A2, thus reducing inflammation in neural tissues and in general.14 Citicoline was shown to have direct free radical suppressive effects, as seen in animal models of transient cerebral ischemia, in which citicoline had a suppressive effect on hydroxyl radical generation.15
Citicoline may significantly impact brain remodeling activity. In an animal model, citicoline treatment significantly increased the length and branch points of dendrites, which led to an increased efficiency of sensory information processing.16 This mechanism of activity could potentially account for a significant portion of citicoline’s neurorestorative functions.
 Clinical Applications
Clinical Applications
Learning and Memory
Experiments in animals and humans provided evidence of citicoline’s ability to promote important cognitive processes, including learning ability and memory functions. Clinical studies evaluating citicoline administration for cognitive enhancement have been conducted for several decades. A review of trials utilizing citicoline as a treatment for senile alterations of memory in 1991 found significant benefits in patients with cerebral insufficiency and chronic cerebrovascular disease.17
A randomized, double-blinded, placebo-controlled study was undertaken to assess the effects of citicoline supplementation on verbal memory function in 95 healthy subjects aged 50 to 85 years (47 women and 48 men) who were administered citicoline (500 mg orally twice daily) or placebo for 3 months. The study subjects were well educated (mean, 14.3 years of education). Baseline testing included a logical memory assessment test, which was used to classify those with relatively inefficient memories. At the end of the initial study, 32 individuals (16 from the citicoline group and 16 from the placebo group) from this pool were recruited to participate in a follow-up crossover study. The initial study found citicoline improved delayed recall for only those with relatively inefficient memory at the beginning of the trial. In the follow-up crossover study, the dose of citicoline was increased to 2000 mg/day. In this subgroup, the higher dose of citicoline improved immediate and delayed logical memory.18
An open-label crossover trial consisting of 24 elderly individuals without dementia, but with demonstrable memory impairment (assessed by comparison with 24 healthy young control subjects), showed that oral citicoline (500, 1000, or 300 mg/day combined with nimodipine [90 mg/day]) significantly improved memory performance compared with the no treatment periods, as evidenced by reduced error scores on word recall tasks, immediate object recall, and delayed object recall.19
Alzheimer’s Disease and Dementia
Citicoline supplementation has been well studied in Alzheimer’s disease and vascular dementia. A study of 19 patients (mean age 66.21 ± 1.48 years) given oral citicoline at a dosage of 1000 mg/day for 30 days found significant improvements in cognitive function in the subgroup of patients with early-onset Alzheimer’s disease and a trend toward increased cognitive function in the overall group, as assessed by brain electrical mapping. Brain spectral data readings provided an indication that the brains of early-onset Alzheimer’s patients showed greater damage than those of late-onset Alzheimer’s patients, whereas both groups had the same degree of cognitive impairment. It was postulated that the therapeutic effects of citicoline might be mediated by an enhancement of cholinergic neural transmission, activation of repair mechanisms to rejuvenate neuronal membranes, a regulatory effect on parameters associated with blood flow and circulation, as well as regulation of several immunologic responses, which, if left unchecked, would lead to potential neuronal dysfunction and cell death.20
In further studies, oral administration of citicoline (1000 mg/day) to 20 patients (age range 57 to 78 years) with early- or late-onset Alzheimer’s disease resulted in improvements in mental function, particularly in the early-onset group. This 1-month treatment with citicoline resulted in an increased blood flow velocity from baseline measures (assessed by transcranial Doppler ultrasound) in the middle cerebral artery, which has been found to decrease with age, possibly resulting in neuropathologic changes. Citicoline’s cholinergic effects and influence on cytokine production might also partially account for its benefits.21
Researchers investigated the regulatory effects of citicoline on blood histamine levels. Alterations in the histamine system are present in Alzheimer’s disease, as high levels have been found in several central nervous system regions, cerebrospinal fluid, and serum. Histamine may also participate in the aging process, with histamine-related changes reported in several different tissues, including the central nervous system. In one study, 14 individuals with Alzheimer’s disease (7 early-onset, 7 late-onset) were administered citicoline (1000 mg/day for 30 days). Blood histamine measurements were taken at baseline, at 2 hours after administration of the first dose, and after 30 days of treatment with citicoline. All participants experienced an acute reduction in blood histamine levels; after 30 days, early-onset Alzheimer’s patients saw a decrease in blood levels of histamine of about 55% compared with baseline, whereas late-onset individuals saw a 45% decrease. Early-onset patients clearly had higher baseline levels of histamine than late-onset patients. Reducing endogenous histamine excesses may support cognitive function, as excessive histamine levels have been implicated in etiopathogenic events in Alzheimer’s disease.22
The effects of citicoline administration (1000 mg/day orally for 3 months) were assessed in a trial in patients with senile dementia (Alzheimer’s disease and multi-infarct dementia) to elucidate whether the nutrient was able to restore immune function and improve mental parameters. The study consisted of four groups: control subjects (n = 8), early-onset Alzheimer’s subjects (n = 11), late-onset Alzheimer’s subjects (n = 7), and multi-infarct dementia subjects (n = 10). After 3 months of treatment, citicoline supplementation improved mental performance in all groups (including controls), as assessed by several standard assessment tools (including the Mini-Mental State Examination and the Hamilton Rating Scale for Depression). Early-onset Alzheimer’s patients showed increased levels of interleukin-1β at baseline. Citicoline administration normalized these levels in the early-onset Alzheimer’s group. The researchers concluded that citicoline showed benefit in senile dementia patients as a restorative and palliative treatment, improving vascular risk factors, stabilizing immune function, and improving mental performance.23 Further studies corroborated these effects of citicoline.24
Citicoline was further studied in a double-blinded, placebo-controlled randomized trial in 30 patients with apolipoprotein-E (Apo-E) genotyped Alzheimer’s disease. All 30 participants were categorized as having mild to moderate dementia. Citicoline (1000 mg/day orally) or placebo was administered daily for 12 weeks, and its efficacy was further evaluated on the basis of each of the individuals’ Apo-E genotype. The development of certain symptoms of Alzheimer’s disease is correlated with differing Apo-E genotypes. The results of the study showed that clinical interview-based impression of change scores worsened significantly in the placebo group, whereas a clear trend toward improvement in the citicoline group was observed. In those individuals bearing the ε4 allele of the Apo-E (Apo-E4), citicoline was found to induce significant improvements on the cognitive function subscale of the Alzheimer’s disease assessment scale. Furthermore, statistically significant improvements in Alzheimer’s disease assessment scale scores were found with citicoline administration in the subset of Apo-E4 patients with mild cognitive deterioration (as assessed by Global Deterioration Scale scores less than 5). An overall increase in cerebral blood flow velocity was also seen with citicoline compared with placebo, whereas beneficial changes were further noted in brain bioelectrical activity.25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree