• Smythe (1972): diagnostic criteria from clinical studies
Obligatory criteria
1. Subjective aching of 3 months or longer
2. Subjective stiffness of 3 months or longer
3. Local point tenderness
4. Point tenderness in two other sites
5. Normal ESR, SGOT, rheumatoid factor, ANF, muscle enzymes, and sacroiliac films
Minor criteria
1. Chronic fatigue
2. Emotional distress
3. Poor sleep
4. Morning stiffness
• Smythe (1979): criteria
History of widespread pain of 3 months or longer
Tenderness at 12 of 14 specified sites
Disturbed sleep with morning fatigue and stiffness
Normal ESR, SGOT, rheumatoid factor, ANF, muscle enzymes, and sacroiliac films
• Yunus (1989): criteria
Diagnosis of primary fibromyalgia syndrome requires major or minor criteria plus obligatory criteria
Obligatory criteria
1. Presence of pain or stiffness or both, at 4 or more anatomic sites for 3 months or longer
2. Exclusion of an underlying condition which may be responsible for the overall features of fibromyalgia
Major criteria
Presence of 2 or more of 6 historical variables, plus 4 or more of 14 specified tender points
Minor criteria
Presence of 3 or more of the 6 historical variables, plus 2 or more tender points
In order to move towards more systematic, empirically driven criteria to classify FMS, a multicenter study (Wolfe et al., 1990) was conducted in the late 1980s, involving approximately 300 patients with FMS and 285 control subjects. The essential point of this study was to delineate factors that could, with good sensitivity and specificity, differentiate FMS patients from people with other chronic pain conditions. Of course, the study was not free from the circular logic problem of FMS diagnosis when trying to determine the eligibility of study patients to define the very disorder of those patients. The multicenter study (Wolfe et al., 1990) dealt with this by defining the 300 FMS patients by the “usual” clinical method that each participating practitioner had been using. Based upon the results, the American College of Rheumatology (ACR) criteria were suggested. FMS patients should present (1) a history of widespread pain of 3 months or longer and (2) the presence of pain responses to at least 11 of 18 designated tender points (TPs). The locations of the TPs are described in Table 6.2 and drawn in Fig. 6.1.
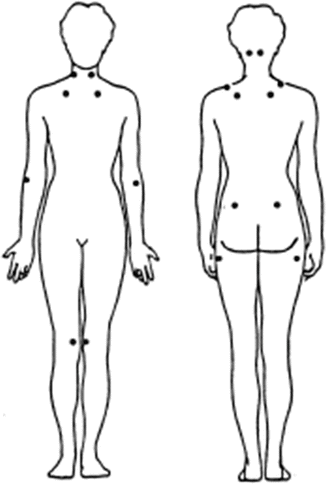
Table 6.2
ACR criteria for classification of FMS
1. Presence of widespread pain for at least 3 months. Pain must be present in all of the body quadrants and axial skeletal area |
2. Presence of pain in at least 11 of 18 tender points on digital palpation with approximately 4 kg force. Tender points are located in 9 bilateral sites as described below |
Occiput: At the suboccipital muscle insertions |
Low cervical: At the anterior aspects of the intertransverse spaces at C5–C7 |
Trapezius: At the midpoint of the upper boarder |
Supraspinatus: At origins, above the scapula spine near the medial boarder |
Second rib: At the second costochondral junctions, just lateral to the junctions on upper surfaces |
Lateral epicondyle: At 2 cm distal to the epicondyles |
Gulteal: In upper outer quadrants of buttocks in anterior fold of muscle |
Greater trochanter: Posterior to the trochanteric prominence |
Knee: At the medial fat proximal to the joint line |

Fig. 6.1
ACR designated tender points
The validity of the ACR criteria, just like the validity of the previously recommended criteria, is difficult to evaluate due to the absence of an absolute “gold standard” for diagnosing FMS, and this leads to a “logic dead end.” The diagnosis is further complicated by the fact that FMS frequently co-occurs with other functional disorders that also are characterized by the symptoms commonly associated with FMS, such as fatigue, sleep disorder, and mood disturbance. Furthermore, since these problems are also common in other chronic pain conditions, they did not show enough discriminating power to be included into the ACR classification criteria. As a result, this exclusion of common clinical complaints has made many wonder how valid the ACR criteria really are (Clauw & Crofford, 2003; Goldenberg, 1999). It is generally granted that the ACR criteria were developed to improve consistency in defining study population. However, the ACR criteria seem to be rarely used in clinical practice. The agreement between FMS classification by the ACR criteria and clinical diagnosis is only modest, with a kappa coefficient of about .5 (Katz, Wolfe, & Michaud, 2006). There are not many disease entities showing such a discrepancy in the diagnostic approach between research and clinical practices.
Although the TP criteria may correspond to the commonly observed hyperalgesic response to experimentally induced noxious stimuli (Clauw, Arnold, & McCarberg, 2011), there is no clear answer as to what painful TPs actually represent. The number of painful TPs (TP counts) is only moderately correlated with clinical pain report (Pamuk, Yesil, & Cakir, 2006), and TPs are generally related to the indices suggestive of psychosocial distress (McCarberg et al., 2003; Wolfe, 1997). In order to respond to the aforementioned criticisms, another multicenter study was recently conducted (Wolfe et al., 2010), yielding the preliminary new diagnostic criteria for FMS. The new criteria quantify CWP and the severity of commonly presented symptoms, but no longer require positive TP counts (see Table 6.2). The symptoms include fatigue, unrefreshed wakening in the morning, and cognitive symptoms. The clinician is also required to rate the extensiveness of somatic complaints. It is important to note that the authors emphasize that the new criteria are not meant to replace the 1990 ACR criteria, but to complement it as a clinical classification tool. Nonetheless, the new criteria are likely to make the integrating CWP and FMS research easier and more meaningful. We have yet to see the data emerging based upon this new FMS classification. Thus, for this chapter, all research data of FMS patients are based upon the 1990 ACR criteria (see Table 6.3).
Table 6.3
New fibromyalgia diagnostic criteria
Criteria that must be met: | |||
1. Other disorders that would explain the pain must be ruled out | |||
2. Symptoms must be present for minimum of 3 months at the stable level | |||
3. Widespread pain index (WPI) and symptom severity scale (SS) levels must be greater than specified as below | |||
WPI: Areas where the patient complain of pain (score 0–19) | |||
Shoulder girdle left | Shoulder girdle right | Upper arm left | Upper arm right |
Lower arm left | Lower arm right | Hip left | Hip right |
Upper leg left | Upper leg right | Lower leg left | Lower leg right |
Jaw left | Jaw right | Chest | Abdomen |
Upper back | Lower back | Neck | |
SS: (sum of severity scores of 3 symptoms and other somatic symptoms) | |||
Severity and symptoms | Fatigue | Waking unrefreshed | Cognitive symptoms |
0: No problem | |||
1: Slight or mild problems, generally mild or intermittent | |||
2: Moderate, considerable problems, often present and/or at a moderate level | |||
3: Severe, pervasive, continuous, light-disturbing problems | |||
Levels of other somatic symptomsa: | |||
0 = No symptoms | |||
1 = Few symptoms | |||
2 = A moderate number of symptoms | |||
3 = A great deal of symptoms | |||
CWP Assessment
Research has used a range of methods to determine the presence of CWP. The determination of CWP requires two parameters of pain: chronicity and multiplicity of pain locations. Typical chronicity is defined as the presence of pain for 3 months or longer. The “widespreadness,” on the other hand, has been defined in various ways. Some used simple descriptions such as “pain all over” or “multiple pain sites.” The two most common standardized methods are the ACR criterion of CWP (see above) and Manchester method (MacFarlane, Croft, Schollum, & Silman, 1996). The Manchester method is more stringent than the ACR method, requiring at least two areas of pain in each limb.
Because the assessment of CWP relies on self-report, often without clinical examination, it is assumed that many of these patients meet the diagnostic criteria for FMS although the degree of such overlap is often not reported. Thus, in this chapter, when the study is only measuring CWP, unless specified, it should be assumed that the sample is likely a mix of patients with CWP but do not meet FMS criteria and patients with both CWP and FMS.
Epidemiology
There is more information about the prevalence of CWP than FMS because CWP can be assessed in a large sample set via survey or interview, whereas FMS requires a physical examination for confirmation based upon the 1990 ACR criteria. The population survey data of 2,000–4,000 community samples in various countries (UK, Sweden, Norway, Israel) revealed a range of prevalence at 4.2–18 %(Abusdal, Hagen, & Bjorndal, 1997; Bergman et al., 2001; Buskila, Abramov, Biton, & Neumann, 2000; Croft, Rigby, Boswell, Schollum, & Silman, 1993; Hunt, Silman, Benjamin, McBeth, & Macfarlane, 1999; Lindell, Bergman, Petersson, Jacobsson, & Herrstrom, 2000; Papageorgiou, Silman, & Macfarlane, 2002). The recent US data with 10,291 community residents (Hardt, Jacobsen, Goldberg, Nickel, & Buchwald, 2008a) yielded a rate of 3.6 % CWP prevalence. The variability in the prevalence rates is at least partially due to the assessment methods each study used. Gerdle et al. (2008) used two methods of assessing CWP: (1) the 1990 ACR CWP criteria and (2) criteria requiring the presence of pain in the spinal region and contralateral limb pain. Of their 7,637 community samples in Sweden, CWP based upon the ACR criteria was reported to be 4.8 % whereas 7.4 % prevalence was attained with the other criteria.
The prevalence rates of FMS also vary across studies, ranging from .7 to 11 % (Forseth & Gran, 1992; Hardt, Jacobsen, Goldberg, Nickel, & Buchwald, 2008b; Prescott et al., 1993; Toda, 2007; Wolfe, Ross, Anderson, Russell, & Hebert, 1995). White, Nielson, Harth, Ostbye, and Speechley (2002) screened 3,395 community residents and found 100 people meeting the FMS criteria (3 %). The National Arthritis Data Working Group has recently estimated that up to five million Americans suffer from this condition (Lawrence et al., 2008).
Although the rates differ across the studies, they consistently report that CWP/FSM is more common in females and have an increased rate with age. The prevalence of CWP/FMS also seems to increase in medical populations, particularly when the condition involves pain. The questionnaire study of 522 patients in the inpatient internal medicine ward with various medical conditions (Buskila et al., 2001) revealed 21 % CWP and 15 % FMS rates. Among 2,730 US workers who were disabled due to work-related injury, 32 % reported CWP (Mayer, Towns, Neblett, Theodore, & Gatchel, 2008). A subsequent study with 449 disabled workers showed a similar rate of CWP (33.9 %), with over two-thirds of those patients also qualifying for FMS. A recent study with 130 chronic back pain patients reported the presence of widespread pain in 28 % of these patients (Nordeman, Gunnarsson, & Mannerkorpi, 2012). These numbers far exceed the prevalence rate in the general public.
Very little is known about the occupational association for the development of CWP/FMS. However, certain occupations may have a higher prevalence of CWP. For example, 14.4 % of 643 female home-care workers reported CWP (Lundberg & Gerdle, 2002), a high-end range of the prevalence reported in the general population studies. Another occupation of interest is military personnel who are often exposed to extremely volatile physical and psychosocial situations. Interviews with veterans of the first Gulf War (Forman-Hoffman et al., 2007) yielded the rate of CWP in18 % of deployed military and 24 % of deployed national guard personnel, significantly greater than nondeployed military (9 %) and national guard (13 %) personnel. Similarly, post-deployment examination of 429 veterans from the Operation Enduring Freedom and Operation Iraqi Freedom yielded a CWP rate of 29 % (Helmer et al., 2009).
Pathophysiology
The etiology of FMS/CWP is unknown. There are several factors, however, that may underlie these conditions. This line of research has been mostly conducted with FMS patients, yielding the potential involvement of both peripheral and central pain modulation, neuroendocrine dysfunction, and dysregulation of the stress system.
Peripheral hypothesis: Earlier studies have suggested that peripheral abnormality in muscles may play a role in FMS. For example, localized hypoxia (Bengtsson & Henriksson, 1989) and metabolic abnormality (Sprott et al., 2000) in the affected areas have been observed in FMS patients. FMS patients may exhibit significantly lower levels of adenosine triphosphate and phosphocreatine based upon the P-31 magnetic resonance spectroscopic analysis of their muscles (Park, Phothimat, Oates, Hernanz-Schulman, & Olsen, 1998), suggesting the presence of muscle weakness and fatigability, possibly associated with metabolic dysfunction of the muscle. However, research has failed to support any electrodiagnostic evidence of ongoing denervation (Durette, Rodriquez, Agre, & Silverman, 1991) or of increased muscle sympathetic nerve discharge (Elam, Johansson, & Wallin, 1992) in FMS. There is also no microscopic evidence of definitive pathology in the muscle tissues of FMS patients (Drewes, Andreasen, Schroder, Hogsaa, & Jennum, 1993). Thus, the pathophysiological involvement of the peripheral abnormality is, at best, inconclusive at this time. The diffuse nature of the pain in this population also provides questionable credibility for the peripheral abnormality hypothesis. Nevertheless, some (Staud, 2011) argue that the potential involvement may occur via forming a peripheral chemical environment that leads to local sensitization, which may contribute to central pain sensitivity.
Central hypothesis: In contrast to the investigation of the peripheral mechanism, research has yielded consistent evidence suggesting the dysregulated central pain modulatory system in FMS. FMS patients exhibit enhanced pain response to various types of experimentally induced noxious stimulation (Arroyo & Cohen, 1993; Gibson, Littlejohn, Gorman, Helme, & Granges, 1994; Kosek & Hansson, 1997; Lautenbacher, Rollman, & McCain, 1994; Petzke, Clauw, Ambrose, Khine, & Gracely, 2003). An imaging study also showed that FMS patients achieved a comparable degree of cortical activation, relative to healthy people, in response to noxious stimuli but at a significantly lower severity of the stimuli (Gracely, Petzke, Wolf, & Clauw, 2002), also suggesting the presence of centrally dysregulated pain modulation in FMS.
FMS patients may also be associated with dysfunction in the endogenous inhibitory system of pain. Attenuated descending noxious inhibitory controls (DNIC) in FMS patients, but not in chronic low back pain patients, were found (Julien, Goffaux, Arsenault, & Marchand, 2005). Significantly reduced DNIC in FMS, relative to healthy people, has recently been reported, and the effects seem to be independent of depression (Normand et al., 2011). Furthermore, FMS patients show increased “windup” (WU) sensitivity (i.e., abnormally heightened temporal summation of pain) and maintain the WU sensitivity (Staud, Price, Robinson, Mauderli, & Vierck, 2004), suggesting the increased excitability of spinal cord neurons related to central sensitization.
Neuroendocrine hypothesis: Another hypothesis regarding the mechanism underlying FMS comes from the studies showing that FMS is related to low levels of serotonin in the plasma (Wolfe, Russell, Vipraio, Ross, & Anderson, 1997), serum (Ernberg, Voog, Alstergren, Lundeberg, & Kopp, 2000), chronic fatigue syndrome (CFS) concentration (Russell, Vaeroy, Javors, & Nyberg, 1992), transfer ratio of tryptophan (Norregaard, Bulow, Mehlsen, & Danneskiold-Samsoe, 1994), and reuptake site density (Russell et al., 1992). FMS may also be associated with disturbance in the dopamine regulation. FMS patients show an increased prolactin response to a buspirone challenge test, suggesting altered sensitivity in dopamine receptors in these patients (Malt, Olafsson, Aakvaag, Lund, & Ursin, 2003). An imaging study, using positron emission tomography tracing L-DOPA uptake, suggests that FMS may be related to the disrupted presynaptic dopamine activity (Wood et al., 2007).
The results from these studies suggest a possibility of the abnormal levels of these neurotransmitters associated with FMS. However, our current understanding of how exactly these neurotransmitters are involved in FMS is limited. Large individual variations in the neurotransmitter levels are present within a group of FMS patients. Clinical correlates of the neurotransmitter levels are also largely unknown. Indeed, the correlations of the serotonin level and depression symptoms in FMS patients were in the reverse direction from the expected in one study (Wolfe, Russell, et al., 1997).
Stress system hypothesis: Given that stress is one of the most prominent aggravating factors for FMS (Okifuji & Turk, 2002), dysregulation of the stress system may be involved in FMS. In general, research supports the notion that FMS may be related to abnormal functioning of the sympatho-adrenal system and hypothalamic-pituitary adrenergic (HPA) axis. FMS patients show altered baseline catecholamines compared to healthy individuals, independent of depression (Hamaty et al., 1989; Loevinger, Muller, Alonso, & Coe, 2007). FMS is also associated with abnormalities of reactivity of the HPA axis, such as abnormal adrenocorticotropic hormone (ACTH) response to exogenous corticotropin releasing hormone (CRH)-induced hypoglycemia and blunted cortisol response (Adler, Kinsley, Hurwitz, Mossey, & Goldenberg, 1999; Crofford et al., 1994). FMS patients also show disturbed heart rate variability (Cohen et al., 2000; Lerma et al., 2011). Compared to healthy people, FMS people show hyporeactive sympatho-adrenal and hypothalamic-pituitary response to exercise (Kadetoff & Kosek, 2010; van Denderen, Boersma, Zeinstra, Hollander, & van Neerbos, 1992). Overall, these studies suggest that FMS is associated with a hyperactive sympathetic activity with hyporeactive response to stress (Di Franco, Iannuccelli, & Valesini, 2010; Martinez-Lavin, 2007). This seemingly paradoxical response may result from chronic hyperstimulation of the beta-adrenergic receptors, leading to receptor desensitization and downregulation (Martinez-Lavin, 2007).
In addition, there may be some genetic components for these abnormalities. A recent study comparing 97 FMS patients to 59 healthy people (Xiao, He, & Russell, 2011) found a specific function-altering beta-adrenergic gene polymorphism in FMS patients, implicating genetic vulnerability in at least some FMS patients. A series of studies investigating gene expression in response to exercise (Light et al., 2012; Light, White, Hughen, & Light, 2009) also demonstrated a significant increase in gene expression of adrenergic molecular receptors in response to exercise, as well as at rest, in FMS patients compared to healthy controls.
Phenomenology of FMS/CWP
FMS/CWP is not lethal or progressive. However, the condition can be quite debilitating, and patients with FMS/CWP report severely compromised quality of life (QOL). QOL is a multifactorial, multilevel concept. In FMS/CWP, not only do disease-related factors determine the QOL, but a number of other psychosocial, environmental, and socioeconomic factors are involved. FMS/CWP seems to significantly influence and interact with those various factors, compromising the QOL for patients with FMS/CWP. For example, a large community survey, investigating comorbid mood disorders in various chronic illnesses (Gadalla, 2008), found that FMS and CFS, which often overlapped, had the highest comorbidity of mood disorders (27 % and 37 %, respectively). Community residents with CWP also seem to have higher comorbid chronic fatigue and mood disturbance (Kato, Sullivan, Evengard, & Pedersen, 2006).
An in-depth interview of eight people with CWP revealed a significant decrement in their ability to manage time in their daily lives due to tasks taking longer and disrupted routines (Richardson, Ong, & Sim, 2008). Natvig, Bruusgaard, and Eriksen (2001) compared 281 chronic back pain patients with widespread pain to 222 back pain patients without CWP and found that the former reported a significant reduction in the QOL, as well as poorer mood. Among people with chronic pain due to work-related injury, those with comorbid CWP/FMS tend to report greater psychosocial stress (Howard et al., 2010). Recently, Nordeman et al. (2012) reported that patients with CWP in the primary care setting exhibited significantly poorer physical functioning, greater pain and fatigue, and more severe mood disturbance than other patients in the practice.
There is little question that FMS/CWP adversely impacts patients’ lives. FMS patients tend to report a lowered sense of physical well-being, greater long-term health concerns (Ejlertsson, Eden, & Leden, 2002; Wolfe, Anderson, et al., 1997a), and increased healthcare utilization (Bombardier & Buchwald, 1996; White, Speechley, Harth, & Ostbye, 1999). FMS seems to be a compounding factor in disability associated with other disease conditions, such as systemic lupus erythematosus (SLE) (Middleton, McFarlin, & Lipsky, 1994). Furthermore, FMS patients tend to rate their QOL as significantly more compromised, compared to other chronically ill patients (Burckhardt, Clark, & Bennett, 1993).
Work-Related Issues and FMS/CWP
Work Disability
Given the multiple symptoms, including chronic fatigue, pain, mood disturbance, and poor sleep, it should perhaps not be surprising that many FMS/CWP patients find it difficult to maintain their productivity at the workplace (Bennett, Jones, Turk, Russell, & Matallana, 2007). Work disability is prevalent in FMS/CWP. Bombardier and Buchwald (1996) reported that 35 % of FMS patients, and over 50 % of FMS patients with concurrent CFS, were unable to be gainfully employed due to their illness. Other studies (De Girolama, 1991; Penrod et al., 2004; Wolfe, Anderson, et al., 1997b) report that work disability due to FMS is found in 9–24 % of FMS patients.
In a study of 91 FMS patients from the community rheumatology clinics (Penrod et al., 2004), 78 % of the patients worked prior to their problems with FMS, whereas 44 % continued to work. Nine percent of the patients reported that they had retired due to FMS, and 16 % were on disability due to FMS. Estimated loss of work time per year due to FMS on average was over 12 weeks. A survey comparison between 136 FMS patients and 152 “clinic controls” (patients recruited from various outpatient clinics) (Al-Allaf, 2007) showed that FMS was associated with greater work dysfunction. Loss of work secondary to the health condition was reported by 47 % of FMS patients and 14 % of clinic controls.
Even when patients are gainfully employed, their productivity and attendance are likely to be adversely impacted by their pain conditions. Accumulated evidence suggests that people with chronic pain, including CWP and FMS, tend to have greater work disability and associated costs for work loss. The data from 31 large self-insured companies in the USA (L. A. White et al., 2008) showed that 8,513 employees with FMS and 7,260 employees with osteoarthritis (OA) missed 30 and 26 days, respectively, per year compared to 10 days in the 7,260 control employees. The cost incurred due to work disability was $2,913 for FMS, $2,537 for OA, and $1,359 for the control subjects (2005 dollars). Winkelmann et al. (2011) surveyed FMS patients in Germany and France and found that, on average, they missed 32 days (France) and 25 days (Germany) of work due to FMS. White and her colleagues, in their systematic examination of 100 FMS patients drawn from the larger community sample, reported 31 % of FMS patients were “work disabled,” whereas 11 % of CWP and 2 % of general controls reported work disability (White et al., 1999). In this study, 65 % of FMS patients, 29 % of CWP subjects, and 9 % of controls reported that they needed to reduce work hours. The leading symptoms that limited work were reported to be pain (87 %), fatigue (80 %), weakness (73 %), and cognitive problems (51 %). In Spain (Rivera, Esteve-Vives, Vallejo, & Rejas, 2011), 68 % of 301 FMS patients reported to have temporary work disability, with the average missing work of 44 days per year. Also, veterans with CWP have a higher likelihood of disability (Forman-Hoffman et al., 2007): Odds ratios of those with CWP to have Veteran’s Administration (VA) disability was 3.14; VA compensation was 2.89; and unemployment due to health problems of greater than 3 months was 7.8.
In a small sample of FMS patients, with the mean age of 43, Martinez, Ferraz, Sato, and Atra (1995) reported that 30 % of their patients had reduced their work hours, and 65 % had a reduction in their family income. A report by Assefi, Coy, Uslan, Smith, and Buchwald (2003) estimates that approximately one-half of patients with FMS lost a job due to the hardship associated with FMS. Even for those currently employed, many patients reduced their working hours (mean hours: 41–45 h per week prior to the FMS onset vs. 31–32 h per week currently). This is not to say that FMS patients willingly terminate their employment. Results from the narrative interview (Liedberg & Henriksson, 2002) indicate that FMS patients consider their work role to be an important part of their self-images. However, patients carefully review the work environment, such as the physical demands of work, the requirement for physical movement, and the opportunity to move around when they evaluate their ability to stay at work. In addition to their physical ability to perform tasks, comorbid conditions, such as fatigue and compromised ability to concentrate, may significantly interfere with certain occupational requirements. Moreover, the availability of psychosocial support at work seems essential. Many FMS patients feel that others do not understand their pain and suffering for their “invisible” illness. Acceptance of their frequent leaves for visiting healthcare providers may also influence their sense of well-being at work.
It is important to note that although FMS is considered as a pain disorder, other symptoms are also important in how occupationally disabled patients perceive themselves. The comparison between working and nonworking FMS patients (Henriksson & Liedberg, 2000) demonstrates that fatigue, irritability, and gastrointestinal discomfort were significant discriminating variables, whereas the two groups did not differ in age, duration of symptoms, number of pain locations, or pain-free time periods. It should also be noted that human resource data (Kleinman et al., 2009), examining the financial burden of illness to the employer, compared various work-related costs among people with FMS, people with OA, and people without FMS (in 2008 dollars). The average costs for short-term disability were $1,706 for FMS, $1,247 for OA, and $263 for non-FMS employees. Both employee groups of FMS and OA showed a greater amount of annual cost associated with sick leave ($582 and $514, respectively), compared to the controls ($329).
Disability Compensation Issues
The difficulty in maintaining gainful employment may lead FMS patients to apply for financial compensation for their disability. The prevalence of receiving financial compensation for their condition varies greatly from study to study. In one study, 55 % of their patients reported to be receiving either temporary or permanent disability compensation (Martinez et al., 1995). In a multicenter study, Wolfe, Anderson, et al. (1997b) found that approximately 15 % of FMS patients receive compensation from Social Security Disability, and an additional 10 % receive other types of financial compensation for disability. The results from a recent Internet survey (Bennett et al., 2007) reported that 20 % of the respondents have a history of filing for disability claims. In a small Swiss study with 48 FMS patients who were followed for 2 years (Noller & Sprott, 2003), 19 % of the patients applied for disability pension. Of course, it is difficult to integrate the results on the disability compensation issues across different countries because of the differences in the entitlement benefit system, laws and regulations governing the disability system, economical strength, and labor market. For example, only a small fraction of disability compensation is provided for FMS in the USA, whereas in Canada, McCain, Cameron, and Kennedy (1989) reported that 9 % of all disability compensation was paid for FMS. The situation, however, may change with a new ruling by the Social Security Administration in July 2012, to be discussed next.
Social Security Disability of FMS in the USA
The Social Security Administration has recently published a ruling providing guidance for the determination of disability claims for FMS patients (Social Security Administration, 2012). Previously, the subjective nature of FMS complaints and the lack of established etiology and pathology made the disability evaluation of FMS patients a difficult challenge. In the new ruling, the medically determinable impairment (MDI) of FMS is now established by appropriate medical evidence gathered by a licensed physician. The evaluation must include the history and physical examination. Treatment notes must be consistent with the diagnosis of FMS and indicate the course of the severity of disease, including the assessment of physical strength and functional abilities for at least 1 year prior to determination. The two sets of criteria acceptable for the diagnosis are the 1990 ACR classification criteria for FMS (Wolfe et al., 1990) and the 2010 ACR preliminary diagnostic criteria (Wolfe et al., 2010). Evidence from “other acceptable medical sources, such as psychologists” may be used in the determination to evaluate the “severity and functional effects of fibromyalgia” or if the person may have another MDI (p. 43643).
Once the MDI of FMS is established, the severity of impairment and whether it prevents the person from work must be determined based upon (a) person’s work activity, (b) severity level of MDI, (c) medical comparability of the person’s impairment with the items in the listing of impairments, and (d) residual functional capacity. Given the newness of the ruling, we cannot ascertain whether the new guidance will change the prevalence of the compensated disability in FMS.
Factors Affecting Loss or Reduction in Work
A number of factors affect a person’s ability to maintain gainful employment. They are not necessarily all clinical factors. Non-clinical factors such as economical trends, physical demands of the job, regional variation in the job market, availability of job accommodations, marketability of patients’ skills, extent of wage potential or replacement if job needs to be changed, and financial incentives all influence the likelihood of FMS/CWP patients staying in the workplace. Once out of the job market, finding an employer may be particularly difficult for patients who have a long history of chronic pain, especially if their job skills are predicated on high degrees of physical exertion. Given the fast-paced changes in technology, some skills can become outdated quickly, even for white-collared workers, thus requiring additional training.
The economic, political, and administrative factors notwithstanding, there are several clinical factors that seem to adversely impact employment and work productivity for FMS/CWP patients. Chronicity of the pain condition, for example, seems to play a role in increasing work disability. In the aforementioned study by White et al. (2002) with 100 FMS patients, the rates of claiming total disability and receiving disability pension were 21 % and 20 % at baseline, and increased to 35 % and 30 %, respectively, over the next 3 years.
The severity of the condition may also be a factor. A recent multicenter study with 203 FMS patients (Chandran et al., 2012) divided those patients into three severity levels, based upon the total score of the Fibromyalgia Impact Questionnaire (Burckhardt, Clark, & Bennett, 1991) that has extensively been used to measure the symptom severity and disability associated with FMS. Almost all of those in the mild range (n = 21) were currently homemakers working outside of the home, whereas this fell to 41 % for those in the severe range (n = 33). Over one-half of the severe range group (54 %) reported to have missed work at least 1 day in the past 4 weeks, whereas 14 % of the mild range group reported any days missed. The aforementioned epidemiological study of mood comorbidity in chronic illness (Gadalla, 2008) suggests that the presence of mood disorder may also be a risk factor for work disability among people with chronic medical conditions; the comorbid mood disorder seems to at least double the risk of short-term disability and may be an additional MDI.
Mannerkorpi and Gard (2012) identified three aspects of work demands: physical, psychosocial, and organizational demands to be critical. In order to best address how FMS patients can maintain their gainful employment, their coping resources to manage all three demands must be present. In their in-depth interview of 27 working FMS patients, limited physical capacity and increased stress were listed as top factors hindering continued work. Thus, modification of one aspect does not necessarily lead to a desirable outcome. For example, simply shortening work days (e.g., from 8 to 4 h a day) does not help much if the psychosocial work demand is unaddressed, as it would maintain the level of stress. Sallinen, Kukkurainen, Peltokallio, and Mikkelsson (2010) also identified four types of work-related concerns from interviewing 20 female FMS workers: mental confusion, coping with fluctuating symptoms, being “in-between” ability to work and disability, and being on the edge of exhaustion. When people are unsuccessful in addressing these concerns, their ability to maintain gainful employment seems to falter over time.
Functional Disability
Functional disability is a significant problem for both CWP and FMS, but particularly in FMS. Functional disability associated with FMS may be comparable in degree to other chronic illnesses, such as rheumatoid arthritis (Martinez et al., 1995) and spondyloarthropathy (Heikkila, Ronni, Kautiainen, & Kauppi, 2002). Others have shown that FMS patients claim a greater degree of perceived disability compared to CWP patients (K. P. White et al., 1999) and spinal cord injury patients (Cardol et al., 2002). In the community sample, having a CWP would significantly predict the likelihood of having low level of physical activity in the future (McBeth, Nicholl, Cordingley, Davies, & Macfarlane, 2010). Functional limitations in CWP/FMS are also observed in performance-based functional testing. Waehrens, Amris, and Fisher (2010) evaluated 50 women with CWP and found their motor and processing skills needed for activities of daily living (ADL) to be significantly reduced. Their subsequent study (Amris, Waehrens, Jespersen, Bliddal, & Danneskiold-Samsoe, 2011) included a larger sample (257 females with CWP) and specified those who met the criteria for FMS (n = 199). The majority of their sample (97 %) exhibited a significantly compromised level of ADL motor skills, with FMS patients showing greater impairment than CWP-only patients. Interestingly, these two studies failed to find meaningful association between performance-based, objective physical functioning, and self-reported disability.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






