Abstract
Objective
Study the indications and level of evidence of clinical exams that might be relevant in exploring the causes of neuropathic pain in spinal cord injury patients.
Method
Literature review from three databases: PubMed, Embase, Pascal.
Results
Disparity and heterogeneity of the answers given by the attendees to the experts conference of the French Society of Physical Medicine and Rehabilitation (SOFMER) and the physicians surveyed via the SOFMER website. These results corroborate the shortage of available data on this topic in the literature. From this analysis, we can however validate spinal MRI imaging as a mandatory exam for the diagnosis of post-traumatic syringomyelia (cystic myelopathy) – this exam can even be considered a Gold Standard. Furthermore, we can also recommend using electrodiagnostic studies for compressive neuropathies. However, it is not possible to validate the relevance of additional clinical exams for radicular pain, segmental deafferentation pain, central deafferentation pain as well as Complex Regional Pain Syndrome (CRPS) type 1; for these types of pain we can only formulate experts recommendations in light of the dearth of available data on the subject.
Conclusion
For the neuropathic pain of spinal cord injury patients’ additional clinical exams should be used in the framework of an etiological diagnosis.
Résumé
Objectif
Étudier la place et le niveau de preuve des bilans paracliniques utilisés pour l’exploration de la douleur neuropathique chez le blessé médullaire.
Méthode
Revue de la littérature à partir de trois bases de données : PubMed, Embase, Pascal.
Résultats
Disparité et hétérogénéité des réponses fournies par les participants à la conférence d’expert et les personnes interrogées via Internet. Ce constat corrobore la pauvreté des données actuelles de la littérature sur ce sujet. De cette analyse, il ressort néanmoins que l’on peut affirmer le caractère indispensable de l’IRM médullaire pour le diagnostic de la syringomyélie post-traumatique cet examen peut être considéré comme un Gold Standard. De même, on peut également recommander l’utilisation de l’étude électrophysiologique dans le diagnostic des mononeuropathies compressives. En revanche, il n’est pas possible de conclure quant à l’intérêt même et à la place des examens complémentaires dans les douleurs radiculaires, les douleurs de désafférentation segmentaire, les douleurs de désafférentation centrale, ainsi que dans le syndrome douloureux régional complexe de type 1, dans ces derniers cas, il n’est possible que de formuler des recommandations d’experts eu égard aux insuffisances de la littérature.
Conclusion
Le bilan paraclinique des douleurs neuropathiques du blessé médullaire ne s’inscrit que dans la perspective d’un diagnostic étiologique.
1
English version
1.1
Introduction
The positive diagnosis (type of pain) of neuropathic pain is based exclusively on clinical data and must be the object of a rigorous clinical exam and patient’s interview; it can be facilitated by using specific and validated diagnostic tools (see question No. 1).
Additional exams (electrodiagnostic studies and MRI mainly) for neuropathic pain in spinal cord injury (SCI) patients should be performed in the framework of establishing an etiological diagnosis. These additional evaluation techniques are presented here according to their IASP classification , i.e. according to the level of injury (below the level of injury, at the level of injury, above the level of injury).
1.2
Material and method
Fifty-eight articles were analyzed and at the end, 18 of them were kept for setting these recommendations. The main analysis criteria included: the clinical exams used, the presence or lack of a Gold Standard exam and finally, if available, the potential prescription hierarchy of additional clinical exams.
The keywords used were:
- •
in the English language: MRI, CT-Scan, electromyogram, syringomyelia, cystic myelopathy, neuropathic pain, spinal cord injury;
- •
in the French language: douleur neuropathique chronique/blessé médullaire traumatique/humain/adulte/traitement non pharmacologique/stimulation nerveuse périphérique/physiothérapie/Tens/acupuncture/hypnose/kinésithérapie/stimulation magnétique transcrânienne .
1.3
Discussion
No article enabled us to directly bring an answer to this question.
1.4
Neuropathic pain above the level of injury
The different reasons for this type of pain are:
- •
compressive neuropathies: such as distal median nerve neuropathies (carpal tunnel syndrome) and ulnar nerve neuropathies;
- •
Complex Regional Pain Syndrome (CRPS) type 1.
1.4.1
Compressive neuropathies
The prevalence of distal median nerve neuropathies (carpal tunnel syndrome) and ulnar nerve neuropathies in SCI patients is respectively 3% and 3.4% . In the case of non-traumatic SCI patients seeking treatment for an aggravation of their neurological disorders (paresthesias, pain, muscle weakness), the incidence rate of carpal tunnel syndrome reaches 34% and ulnar neuropathies 23% . The frequency of these neuropathies increases with the time elapsed since the initial trauma .
Electrodiagnostic studies are today the most used clinical tools enabling to: confirm nerve lesions, localize the lesion, evaluate the severity of the disorder. However, the analysis of the literature does not yield any specific study on the relevance of electrodiagnostic studies for compressive (entrapment) neuropathies in SCI patients. In non-SCI patients, the American Academy of Electrodiagnostic Medicine (AAEM), the American Academy of Neurology (AAN) and the American Academy of Physical Medicine and Rehabilitation (AAPM&R) recommend as a first treatment to measure the sensory conduction velocity (SCV) of the median nerve through the carpal tunnel, over a distance of 13 to 14 cm, between the wrist and finger (antidromic); in case of abnormalities, it is proposed to compare this velocity with the one recorded on another nerve of the same limb (in order to rule out any diffuse process). For median SCV, the sensitivity is rated at 65% and specificity at 98%. Another report study (meta-analysis) from the Agency for Healthcare Research and Quality (AHRQ) in 2002 rated the median SCV with a sensitivity at 76% and a specificity at 96%.
On the contrary, if the distance is superior to 8 cm and median SCV is normal, other electrodiagnostic study techniques are suggested and performed in a ranking order.
If there is a presumption of ulnar neuropathy at the elbow the AAEM recommends first to record motor conduction velocity (MCV) (stimulation above, and below the elbow and at the wrist with a recording of the muscle evoked response on the abductor digiti minimi ) or the SCV. In case of abnormal MCV or SCV recordings, it is recommended to study the sensory conduction on another nerve of the same limb (to rule out any diffuse process). In case of inconclusive results, other electrodiagnostic study techniques are suggested and should be performed in a ranking order.
There is no mention in the literature of the mechanisms causing these compressive neuropathies.
The literature does not report any data on the thoracic outlet syndrome (TOS).
1.4.2
Complex Regional Pain Syndrome (CRPS) type 1
The number of publications devoted to CRPS type 1 in SCI patients is quite low (11 studies found between 1984 and 2007). Furthermore, often the studies, essentially retrospective, are reporting only one unique case or focusing on a small cohort ( n ≤ 8). Only one prospective study including 60 patients reports a 10% prevalence .
CRPS type 1 occurs in the months following the injury and generally affects in an unilateral manner one upper limb in quadriplegic patients; bilateral affections of the upper limbs and affections of the lower limbs were very rarely reported.
In the various published studies the diagnostic criteria were quite disparate.
In 1994, the IASP validated four diagnostic criteria based on clinical arguments exclusively and this regardless of the CRPS type 1 etiology ( Table 1 ). Since these criteria were rarely used, in 2003, new diagnostic criteria (still based on clinical data only) were proposed and are awaiting validation by the IASP .
| A syndrome that develops after an initiating noxious event |
| Spontaneous occurrence of pain in theabsence of an external stimulus, allodynia (pain due to a mechanical or thermal stimulus that normally does not provoke pain), or hyperalgesia (exaggerated response to a stimulus that is normally painful) that is not limited to the territory of a single peripheral nerve, and is disproportionate to the inciting event |
| There is or has been evidence of edema, skin blood flow abnormality, or abnormal sudomotor (sweating) activity in the region of the pain since the inciting event |
| This diagnosis is excluded by the existence of conditions that would otherwise account for the degree of pain and dysfunction |
No data were found in the literature on the relevance and ranking orders of additional clinical exams (comparative X-rays, 3-phase bone scintigraphy, MRI imaging…) for validating the CRPS type 1 diagnosis. Furthermore and for the time being, we do not have any Gold Standard exam for the diagnosis of CRPS type 1 .
Due to the lack of specific diagnostic markers, only an array of various anamnesis data, clinical signs and eventually additional specific exams can confirm the diagnosis.
1.5
Neuropathic pain
Including:
- •
post-traumatic syringomyelia (cystic myelopathy);
- •
radicular pain (spinal root nerve compression);
- •
segmental pain (transitional zone pain felt at the level of injury).
1.5.1
Post-traumatic syringomyelia (PTS) (cystic myelopathy)
The prevalence of PTS is difficult to assess for many reasons: most studies date back from before MRI use, diagnosis based on clinical data or X-rays exams, neurological definition of the syrinx. Thus, this prevalence varies between 0.6 and 3.2% in the old studies compared to 3.5 to 28% in the most recent cohort studies . The delay of onset varies from a few weeks to several years (2 months to 30 years) after the initial trauma .
In a recent prospective study, the prevalence of PTS-related pain is estimated at 75% at 5 years .
Magnetic Resonance Imaging (MRI) remains the Gold Standard exam for syringomyelia diagnosis. It enables to: see the cavity, analyze precisely its diameter (width and length), look for septa. It must include: T1 and T2 weighted images, sagittal entire cord images in addition to sagittal and axial slices centered on the site of injury, eventually T2-weighted sequences for studying small cavities . Syringomyelia is translated by an increased cord signal (similar to CSF) with low-density T1-weighted image and high-density T2-weighted image.
In practice, we need to differentiate :
- •
the cyst: intramedullary cyst with well-defined margins, which is eccentric, extends beyond the limits of the original cord injury, does not communicate with the central canal or the fourth ventricle, and is isodense with CSF (i.e., low-density T1-weighted image, high-density T2-weighted image) is characteristic;
- •
syrinx: is an intramedullary tube-like lesion with one (or both) usually tapering cephalad or caudad ends, well defined margins, which extends at least to two levels to be qualified as syrinx .
Coupling the morphological imaging to a velocity study (pericystic and intracystic CSF velocity) does not benefit yet from a systematic indication.
Alternate imaging exams are justified only for patients with an MRI contra-indication (cardiac pacemaker, neurostimulation device, ocular metallic foreign body). In that case, a CT myelogram (combine CT scanning with myelography) is the alternative: 5 mm slices every 15–20 min, performed at 6, 18 and 24 h after injecting the product (due to the random spreading of the contrast dye in the cavity after the injection) .
Studies regarding the relevance of evoked potentials are methodologically insufficient (case studies, simultaneous inclusions of communicating syringomyelia and non-communicating syringomyelia, lack of a clear demonstrated benefit during the pre and post-surgical studies).
1.5.2
Radicular pain (nerve root compression)
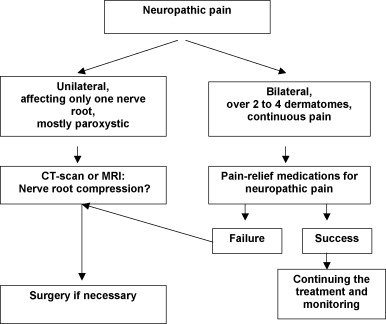
Radicular pain is caused by nerve root damage around the area of injury (the area of injury being, by definition spread at the maximum over two dermatomes above and below the neurological level); radicular pain is usually located on one side only, it is often paroxystic and can be associated to mechanical instability of the spine .
The literature analysis does not bring any precise information on additional exams to be performed. However, it seems relevant to recommend performing a CT-Scan or MRI to verify the localization of surgical implants (screws or clips) and to look for a nerve root compression in the foramen caused by a broken piece of bone, dislocated disc material or inflammation ( Fig. 1 ).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






