Abstract
Objective
Chronic pain is very frequent after spinal cord injury, recent data showing that at least 80% of the patients experience pain, one-third at a severe level. The main objective of the present work is to report and discuss data regarding tools and procedures for the screening, diagnosis, and evaluation of neuropathic pain in spinal cord injury patients.
Material and method
The method used is that developed by the SOFMER, which associated a systematic review of the literature and a selection of published works by a scientific commitee, an analysis of data performed by a binom neuropathic pain/physical medicine and rehabilitation (PM&R) specialists, an evaluation of current practices during an expert consensus conference and via Internet, and finally a validation of the whole work by a pluridisciplinary expert panel.
Results
The literature provides an important series of studies on pain in spinal injury, but without specific data about neuropathic pain in this population. Some specific diagnostic and evaluation tools for neuropathic pain have been developed these last years, while numerous classifications, based on various criteria, have been proposed, some of them exhibiting some advantages for a pragmatic application and being in parallel in accordance with recent nosological and physiopathological advances.
Discussion
The DN4 questionnaire can be used for the screening and identification of neuropathic pain in this population of patients, often suffering from various types of pain. The use of the Spinal Cord Injury Pain Task Force of the International Association of the Study of Pain classification (SCIP–IASP), although some limitations, is recommended since taking into account physiopathology, localisation, and nature of pain. Daily uses of Visual Analogic Scale (VAS) or Numeric Scale (NS) are an obvious need and that of the questionnaire Douleur de Saint-Antoine (QDSA) for global evaluation and more specifically of the Neuropathic Pain Symptom Inventory (NPSI) for neuropathic pain are highly recommended.
Résumé
Objectif
La douleur chronique est fréquente chez le blessé médullaire, les données les plus récentes rapportant qu’au moins 80 % des patients sont douloureux chroniques, dont un tiers de façon sévère. L’objectif du présent travail est de rapporter et discuter de façon pratique les données concernant les outils et procédures d’identification, classification, et évaluation des douleurs de type neuropathique chez le blessé médullaire.
Matériels et méthodes
La méthode utilisée est celle développée par la Sofmer, associant une revue systématique de la littérature et une sélection des travaux publiés établie par un comité scientifique, l’analyse des données réalisée par un binôme neurologue–algologue/médecin de médecine physique et réadaptation, un recueil des pratiques professionnelles effectuée par vote au cours d’une conférence d’experts et via Internet, puis une validation finale par un panel pluridisciplinaire d’experts.
Résultats
La littérature fournit une importante série de publications à propos de la douleur chez le blessé médullaire, mais l’absence de données spécifiques concernant la douleur neuropathique dans cette population est remarquable. Quelques outils diagnostiques et d’évaluation spécifiques de la douleur neuropathique ont été développés ces dernières années, sans référence particulière à une étiologie donnée. En revanche, de nombreuses classifications, basées sur des critères très divers, ont été proposées, dont seulement quelques-unes ont un aspect pratique évident tout en faisant référence aux données nosologiques et physiopathologiques les plus récentes.
Discussion
Le questionnaire d’aide au diagnostic DN4 doit être utilisé dans l’identification spécifique de la douleur neuropathique dans cette population de patients arborant souvent plusieurs types de douleur. L’usage de la classification de la Spinal Cord Injury Pain Task Force of the International Association of the Study of Pain (SCIP–IASP), malgré certaines limites, est recommandé, car elle tient compte à la fois de la physiopathologie, de la localisation, et de la nature de la douleur. L’usage au quotidien de l’échelle visuelle analogique (EVA) ou de l’échelle numérique (EN) est évident et celui du questionnaire Douleur de Saint-Antoine (QDSA) pour l’évaluation globale et plus spécifiquement du Neuropathic Pain Symptom Inventory (NPSI) pour la douleur neuropathique sont également recommandés.
1
English version
1.1
Introduction
Chronic pain is quite commonly found in spinal cord injury (SCI) patients, with a reported prevalence from 11 to 94% , the latest data from the literature report at least 80% of SCI patients suffering from chronic pain and one third of them qualify this pain as severe . Chronic pain has a significant negative impact on sleep and quality of life, it has rarely been evaluated in older studies, or only in relation to its intensity, and lately to its impact using multidimensional evaluation tools .
The prevalence of chronic pain in SCI patients appears to be increasing greatly, as reported by the latest data from the literature. However, these data are hard to analyze since several studies are transversal, sometimes retrospective, by questionnaire or postal survey, and rarely discriminating regarding the type of pain: neuropathic or nociceptive.
The aim of this work is to review the available data regarding the tools or procedures for identifying, classifying or evaluating the neuropathic pain in SCI patients.
1.2
Material and method
The methodology used, proposed by the French Society of Physical Medicine and Rehabilitation (SOFMER) , includes a systematic review of the literature, the gathering of information regarding current clinical practices and a validation by a multidisciplinary panel of experts. The systematic review of the literature and the studies selection were done by the scientific committee, then the data analysis was conducted independently by two readers using the blinded method. These two physicians are from two different medical specialties, one is a neurologist and chronic pain management specialist and the other one is a PM&R specialist.
An identification of the common professional practices was conducted during the Experts Conference at the SOFMER meeting. The vote included 116 physicians who attended the conference and 50 others who answered via the SOFMER website. This vote consisted of answering three questions regarding the validated criteria for the neuropathic pain diagnostic, the classification tools for the SCI patients’ pain and the evaluation tools for chronic pain, specifically neuropathic pain.
1.3
Results
1.3.1
Identification
Based on the data gathered form the literature, we can consider that neuropathic pain is an important part of the chronic pain in SCI patients, at least 40% according to Norrbrink-Budh et al. , this pain is often hard to identify with a late diagnostic, whereas nociceptive pain, which is common and increased by movement (SCI segmental pain at the level of injury, spasm, joint pain) is well-known and easier to identify. In fact, one single SCI patient, can be affected by different types of pain, neuropathic and nociceptive, located in several areas , in 70% of cases the neuropathic pain was located below injury level .
1.3.1.1
Characteristics of neuropathic pain
The semiological characteristics of neuropathic pain and its description vary from one study to the next. The literature reports three main types of symptoms.
1.3.1.1.1
Spontaneous pain
- •
Continuous: burning or painful cold sensation, stinging, tearing, bursting, stabbing, pressure sensations;
- •
paroxysmal: electric shocks sensation.
1.3.1.1.2
Provoked pain
- •
Allodynia: pain due to a stimulus which does not normally provoke pain;
- •
hyperalgesia: an abnormally increased response to a stimulus which is a little painful.
The burning sensations are the ones most often reported in the literature . This burning pain can be superficial or deep, most often found below the level of injury. This pain can have an early onset, a few days after the initial injury, but it can also appear later on, mainly during the first two months . Many factors were studied in order to identify determining or predictive factors for this type of pain, but due to the lack of prospective longitudinal studies, no significant factor can be validated .
These two types of pain are often associated to continuous pain, with a higher frequency in the areas where there is a thermal sensory impairment .
1.3.1.1.3
Sensations that do not provoke pain
Beyond pain, we can find painless sensations, unpleasant or not, that are more often paresthesias, reported sometimes with the improper term “phantom pain” . Paresthesia is an abnormal sensation, whether spontaneous or evoked, neither painful, nor unpleasant, often with prickling or tingling sensation but that can bother the patient.
Paresthesia or “phantom pain” in SCI patient is quite common, with an early onset, and tend to disappear over time. It is characterized by painless but complex sensations in terms of position, size, shape, or movement of the deafferented limbs . It is quite similar to spontaneous pain in terms of characteristics, description, intensity, delay of onset (60% in the first 6 months), and impact, but it is less common and located essentially in the lower back and lower limbs . It is often associated to provoked pain .
1.3.1.2
Neuropathic pain diagnostic
Due to the lack of validated diagnostic criteria, identifying neuropathic pain in SCI patients suffering from chronic pain is difficult and must be based on solid facts, above all clinical symptoms, and a precise anatomical knowledge of the neurological level of injury . The neuropathic pain diagnostic must be first based on the patient’s interview and clinical exam, no additional exams (X-rays, MRI, etc.) should be necessary to evaluate the neuropathic nature of the pain . Additional exams are only relevant for the etiological diagnostic of the injury causing the pain or to evaluate the positive diagnostic of neuropathic pain (see below).
When a patient presents a combination of several typical pain syndromes and sensory impairments, the probability for neuropathic pain is high. Some symptoms or basic impairments bring up the notion of neuropathic pain and are thus discriminating elements for validating the neuropathic nature of a painful syndrome :
- •
painful symptoms:
- ∘
sensation of a superficial continuous burning pain,
- ∘
sensation of painful cold,
- ∘
spontaneous segmental electric shocks,
- ∘
allodynia to skin brushing,
- ∘
allodynia to cold;
- ∘
- •
painless symptoms:
- ∘
continuous and systematic distal paresthesias;
- ∘
- •
any sensory impairment located in a painful area.
If the presence of only one symptom can bring up the notion of neuropathic pain, no pain symptom by itself is a pathognomonic validation for this type of pain: its the combination of different pain symptoms and sensory impairments, at least within the same neurological area, that constitutes the basis for the diagnostic of neuropathic pain . In practice, elementary symptoms and clinical signs that are systematically found at the level of injury and/or below the level of injury, are essential and should bring up the notion of neuropathic pain. But they are not mandatory for the neuropathic pain diagnostic: various symptoms can coexist at the level of injury or below and in some cases, usually with an old injury, the pain symptoms can extend beyond the sensory impaired area.
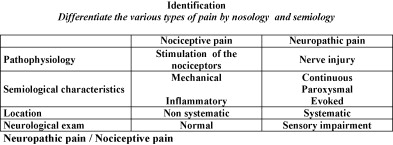
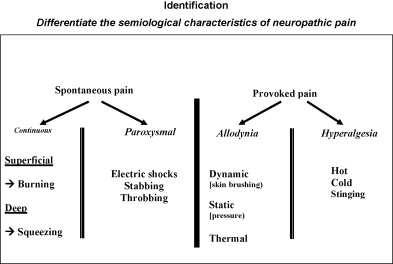
The semiological characteristics of nociceptive or neuropathic pain are reported in Fig. 1 , the definition of elementary symptoms encountered in neuropathic pain is reported in Fig. 2 , and the most common associations of elementary symptoms and neuropathic pain are reported in Fig. 3 .

1.3.1.3
Questionnaires for neuropathic pain diagnostic
1.3.1.3.1
Leeds Assessment of Neuropathic Pain Scale (LANSS)
This questionnaire, the first one designed for neuropathic pain diagnostic, was not completely validated or translated into French , including its most recent shortened version (S-LANSS). It is based on questions asked to patients associated to a clinical evaluation conducted by the investigator. This diagnostic tool has several drawbacks. On the one hand, there are too many items linked to clinical affections that are borderline to neuropathic pain, like Complex Regional Pain Syndrome (CRPS). On the other hand, the questions asked to patients are not specific to each of the validated characteristic of neuropathic pain, such as allodynia or continuous spontaneous pain, but rather they cover several descriptions in one single item, making the intrinsic value of each item vary from one patient to the next. Furthermore, the fundamental multidimensional nature of some descriptive items, with a low diagnostic value in the framework of neuropathic pain, positions this questionnaire on two different levels : diagnostic and evaluation. Its sensitivity and specificity do not seem sufficient to validate, with a good reliability level, the clinical elements of neuropathic pain, compared to other chronic pain syndromes.
1.3.1.3.2
Neuropathic Pain Questionnaire (NPQ)
It is a self-administered questionnaire that lists several items, including some that are not neuropathic pain-specific as they are linked to an emotional response or the changes affecting the pain sensation in relation to various outside events . This questionnaire does not include a sensory exam of the patient. In practice, the sensitivity at 66% and specificity at 74% justify using this tool for chronic pain patients when the neuropathic origin of the pain is already highly suspected.
1.3.1.3.3
DN4 questionnaire
The only diagnostic tool that is strictly clinical and validated is the DN4 questionnaire, with the potential to obtain a clinical score and compare it to a threshold, the specificity for detecting neuropathic pain is evaluated at 82.9% and sensitivity at 89.9% . This diagnostic tool designed and validated by a group of French experts is a 10-item questionnaire divided into seven questions for the patient and three items related to the clinical conducted by the physician ( Table 1 ). Each item in this questionnaire was selected because it brought up the notion of neuropathic pain based on a comparative study between two populations of patients (neuropathic pain vs. arthritic pain), and the diagnostic value of their combination was also evaluated. The presence of at least four out of ten subjective symptoms or objective signs brings up the neuropathic nature of the pain syndrome. A 7-item interview shortened version was extracted, with a 3-item threshold value. The DN4 questionnaire cannot establish that the pain is solely neuropathic: it can confirm, with a high level of reliability, the notion of a neuropathic component to the chronic pain affecting the patient. It is thus a very easy to use but highly discriminating tool, for establishing a neuropathic pain diagnostic in spinal cord injury patients.
| Neuropathic pain DN4 Questionnaire |
| Answer the 4 questions below with yes/no for each item : |
| Interview |
| Question 1: does your pain present one or more of the following characteristics |
| 1 Pain feels like burning |
| 2 Sensation of painful cold |
| 3 Pain feels like electric shocks |
| Question 2: in the same area, is your pain associated to one or more symptoms |
| 4 Tingling |
| 5 Prickling (picks and needles) |
| 6 Numbness |
| 7 Itching |
| Clinical exam |
| Question 3: is the pain located in an area where the exam unveils |
| 8 Hypoesthesia to contact |
| 9 Hypoesthesia to pricking |
| Question 4: Is the pain provoked or increased by |
| 10 Brushing |
1.3.1.3.4
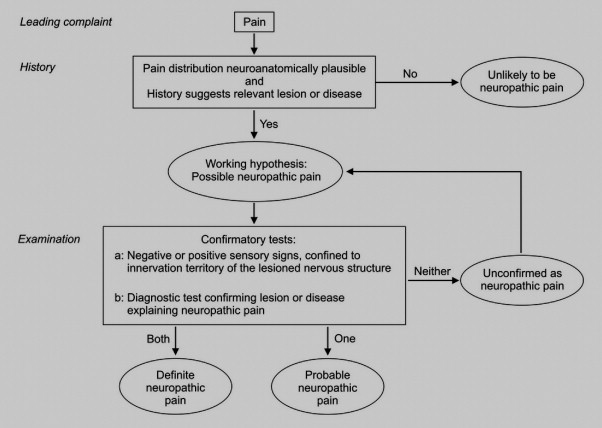
Neuropathic Pain Specific Interest Group (NeuPSIG) grading system
A grading system for refining the positive diagnostic of neuropathic pain has been recently proposed by the Neuropathic Pain Specific Interest Group (NeuP-SIG) of the International Association for the Study of Pain (IASP) . It is a data collection with the following bases:
- •
the existence of a relevant lesion anatomically defined;
- •
the existence of a sensory impairment at the level of injury;
- •
the existence of pain symptoms at the level of injury and eventually the areas around it;
- •
diagnostic test confirming lesion or diseases explaining neuropathic pain.
This grading system ( Fig. 4 ) is quite relevant on a nosological and taxonomic level. However, it is quite hard to use in daily clinical practice, afar from specialized pain management centers were specific additional exams can be performed.
1.3.1.4
Semiological description
Siddall and Loeser report a number of clinical pain syndromes in SCI patients (spinal cord mechanical instability, painful muscle spasms, overuse or pressure pain syndromes, compressive neuropathies, syringomyelia pain, segmental or transitional zone pain, central dysesthetic pain) and take into account the so-called psychological “painful” syndromes. This description was followed by a more mechanical definition of the pain. Thus in SCI patients, it is essential to differentiate the neuropathic pain directly related to the injury from the one not directly related to the injury :
- •
chronic, neuropathic pain from spinal cord injury;
- •
neuropathic pain with a double nature, peripheral and central, often acute and that can become chronic (example: radicular pain at the level of injury);
- •
compressive neuropathies, with probably a dual aspect, neuropathic and inflammatory, they have an acute onset but can become chronic (example: carpal tunnel syndrome), most of the time due to an overuse, and they can be found above the injury level;
- •
provoked pain, for which the pathophysiological mechanism is most probably neuropathic, but the diagnostic criteria does not fit and it should be considered as a subtype of provoked paroxysmal pain.
1.3.1.4.1
Central neuropathic pain located below the level of injury
Central neuropathic pain located below the level of injury (before improperly called phantom or deafferentation pain), spontaneous or provoked, is located below the level of the injury, most often felt as a burning pain or electric shocks, with hyperalgesia, it sets in quite quickly after the initial injury. This pain can fluctuate according to various factors. It is often associated to allodynia in incomplete SCI. Its prevalence is evaluated at 47% .
1.3.1.4.2
Neuropathic pain at the level of injury
Neuropathic pain at the level of injury (transitional zone pain or segmental pain) is usually felt at the level of injury in a band-like pattern around the trunk over two to four dermatomes. This pain is often associated to allodynia and hyperalgesia; the pain is either linked to nerve root damage and is unilateral, or to an injury of the posterior horn and is then bilateral. Its prevalence was estimated at 19%, and its association to central pain at 22% .
There are several specific cases.
1.3.1.4.3
Pain linked to a syrinx cavity
Pain linked to a syrinx cavity should be considered according to its location (above, below or at the level of injury) but is also an integral part of the neuropathic pain in a given patient since the distinction between what is caused by the SCI itself and what is triggered by the syrinx cavity is very difficult and almost impossible to determine in practice. This pain is often characterized by an increase of the sensory impairments right above the injury.
1.3.1.4.4
Cauda equina pain and pain caused by a low-level injury
Cauda equina pain and pain caused by a low-level injury includes a peripheral aspect since it affects the most distal metamers or the nerve roots.
It is important to consider the completeness of the injury (incomplete or complete SCI) as well as the topography of the injury somatosensory pathways, dorsal column or spinothalamic, both the conditions and the pain’s semiology are still being further investigated to this day.
Finally, we can see patients affected by neuropathic pain that is not SCI-specific , but rather the consequence of peripheral neurological lesions caused by overuse, and is located above the level of injury.
1.3.1.5
Differential diagnostic
It is a differential diagnostic that should be based on comparisons with other types of pain affecting SCI patients:
- •
musculoskeletal pain:
- ∘
the pain is often acute, affects the bones, ligaments, muscles or joints, is located in a precise area and sensitive to physical exercise; or chronic with an overuse of the upper limbs in paraplegic patients: sometimes with muscle spasms in incomplete SCI ;
- ∘
- •
visceral pain:
- ∘
nociceptive pain is most often acute, hard to evaluate depending on the level of the injury, with vague, uncharacteristic and unpleasant sensations in the painful area but also felt as projected pain sensations;
- ∘
- •
CRPS:
- ∘
its classification is being argued, since the semiological characteristics are partly neuropathic but also with vascular or dystrophic components, most often affecting the upper limbs of tetraplegic patients, at the level of injury or below .
- ∘
1.3.1.6
Remarks regarding the questionnaire tools
The advances in the field of understanding neuropathic pain led to the design of diagnostic tools. The DN4 questionnaire designed in French language is a validated and reliable tool to use in daily clinical practice, for specialists or non specialists. This regardless of the neurological injury, its pathology, and the underlying mechanisms triggering the neuropathic pain and its various clinical symptoms.
1.3.2
Classification
1.3.2.1
History
Many classifications systems have been suggested, and already 29 of them were reported by Hicken et al. in 2002 . Historically, classification modalities have progressed since the basic classifications that used to describe the pain’s characteristics, its location and its pathophysiology . Progressively new classifications came up that were designed according to location criteria (above, below or at the level of injury) and according to the pain’s pathophysiology (central neuropathic pain or radicular neuropathic pain, nociceptive pain, visceral pain).
1.3.2.1.1
Classification according to the pain’s description
This classification is based on the subjective pain experience described by the patient and is frequently used even though, it is not objective.
1.3.2.1.2
Classification according to the pain’s origin
This is often how the studies’ results are reported, mainly when using questionnaires. This classification is based on the notion of the pain’s origin, and includes all types of pain. This classification is unreliable since the pain’s action mechanisms are not necessarily determined. For example, it is has been suggested to differentiate the various types of pain: radicular pain, segmental pain, visceral pain, phantom pain and cauda equina pain syndromes.
1.3.2.1.3
Classification according to the pain’s location
This is the classification most often used, according to the injury level (above, below or at the level of injury). However, it is often difficult to use since various pain etiologies are found at the same level of injury. At the level of injury, we find nociceptive back pain and neuropathic SCI pain, at the level of injury we find visceral and neuropathic pain and above the level of injury we find visceral pain, nociceptive pain (muscle and joint pain), neuropathic pain due to overuse or autonomic dysreflexia pain.
Beyond these common classifications, several authors have suggested some new classifications frequently found in the literature.
1.3.2.1.4
Donovan pain classification
Donovan pain classification is essentially based on the pain’s terminology: segmental pain, spinal cord pain, visceral, pain, mechanical pain and psychogenic pain. It was modified by Loubser and Donovan, by introducing two categories, neuropathic and non neuropathic, and subcategories, respectively segmental, central, visceral, musculoskeletal and psychogenic .
1.3.2.1.5
Tunks pain classification
It identifies 11 pain types :
- •
above the level of injury: myofacial, syringomyelia, non spinal cord;
- •
at the level of injury: radicular, hyperalgesia at the level of injury, fracture pain, myofacial pain (incomplete injury);
- •
below the level of injury: diffuse burning pain, phantom pain, visceral pain, myofacial pain (incomplete injury).
1.3.2.1.6
Christensen and Jensen pain classification
It groups together five main pain types: radicular pain, segmental pain, diffuse pain or phantom pain, visceral pain and non neurological pain, allodynia and dysesthesia. . It is not designed for a practical use, especially in terms of defining a precise diagnostic for specific pain care management.
1.3.2.1.7
Siddall pain classification
This classification identifies various characteristics for qualifying the pain, both on a pathophysiological level and a topographic one . This classification underlines three successive levels that can characterize the pain:
- •
level I: pathophysiology of the pain: musculoskeletal pain, visceral pain, neuropathic pain, other type pain;
- •
level II: only for neuropathic pain: pain location according to the level of injury (at, above or below the level of injury);
- •
level III: only for neuropathic pain: type of pain (radicular pain or central pain).
1.3.2.1.8
Bryce–Ragnarsson pain classification
Evolution of a classification originally proposed by Ragnarsson , modified later on ( Table 2 ), it includes 15 pain types and some subtypes for the location according to the level of injury, the nociceptive or neuropathic nature and the etiology .
| Bryce–Ragnarsson Sci Pain Taxonomy, 2001 | |||
|---|---|---|---|
| Location | Type | Etiologic subtype | |
| Above level | Nociceptive | 1 | Mechanical/musculoskeletal |
| 2 | Autonomic dysreflexia headache | ||
| 3 | Other | ||
| Neuropathic | 4 | Compressive neuropathy | |
| 5 | Other | ||
| At level | Nociceptive | 6 | Mechanical/musculoskeletal |
| 7 | Visceral | ||
| Neuropathic | 8 | Central | |
| 9 | Radicular | ||
| 10 | Compressive neuropathy | ||
| 11 | Complex regional pain syndrome | ||
| Below level | Nociceptive | 12 | Mechanical/musculoskeletal |
| 13 | Visceral | ||
| Neuropathic | 14 | Central | |
| 15 | Other | ||
1.3.2.1.9
Cardenas pain classification
Proposed in 2002 , it lists two major categories: neurological and musculoskeletal pain. The neurological pain category is further divided into four subcategories: SCI pain, transition zone pain, radicular pain and visceral pain). The musculoskeletal category is further divided into subcategories: mechanical SCI pain and overuse pain.
1.3.2.2
The latest pain classification
All the various systems listed above were the groundwork for elaborating a new classification proposed by the International Association of the Study of Pain (IASP).
1.3.2.2.1
Spinal Cord Injury Pain Task Force of the International Association of the Study of Pain: SCIP–IASP pain classification
It was proposed in 2000 and has become a reliable reference tool but remains to be worked on for validation, ongoing updates but also critical comments ( Table 3 ). It identifies three classification levels:
- •
a first level for the pathophysiological pain type: nociceptive or neuropathic pain, according to IASP definitions;
a second level for the nociceptive pain identifying the affected system, musculoskeletal or visceral pain, and for the neuropathic pain identifying the location according to the level of injury;
a third level, identifying the specific structures or pathologies potentially involved.
| Spinal Cord Injury Pain Task Force of The IASP, 2000 | ||
|---|---|---|
| Type | System | Affected structures/Pathologies |
| Nociceptive | Musculoskeletal | Bone, joint, muscle, |
| Mechanical instability | ||
| Muscle spasm | ||
| Overuse syndrome | ||
| Visceral | Renal lithiasis (kidney stones) | |
| Digestive pathology | ||
| Sphincter dysfunctions | ||
| Headache by AD | ||
| Neuropathic | Above level | Tunnel syndromes |
| CRPS | ||
| at level | Radicular compression (cauda equina) | |
| Syringomyelia | ||
| SCI trauma/ischemia | ||
| Double-injury syndrome (spinal cord + nerve root) | ||
| Below the level | SCI trauma /ischemia | |
It is interesting to note that psychogenic pain is not included in this classification. The cognitive, emotional and environmental factors should be considered as promoting factors when the pain is chronic. IASP underlines that psychological factors are an integrated part of any chronic pain syndrome. The existence of psychogenic pain is argued in the specific context of a spinal cord injury, yet there can be a sine materia pain occurring in a framework of psychiatric disorders.
1.3.2.3
SCIP–IASP classification limits
Some limits to the SCIP–IASP classification were reported and the SCIP–IASP was benchmarked to the most recent classifications.
1.3.2.3.1
Regarding the SCIP–IASP classification in incomplete SCI
The distinction neuropathic/nociceptive can be quite problematic since the semiology of the nociceptive pain occurring in a partially deafferented area can be misleading. Furthermore, neuropathic pain can also be the mirror image, at a given moment, of a suffering linked to or associated to a functional pathology, close to the level of injury or at a distance (hyperactive bladder, urinary tract infection, inflammatory syndrome). For some others authors , visceral pain is classified with the nociceptive pain by IASP, but when there is chronic abdominal pain without any evidence of a visceral pathology, it can justify its classification with neuropathic pain. Furthermore, autonomic dysreflexia (AD), including headaches is included with visceral pain.
1.3.2.3.2
Regarding the inter-user reproducibility when using these classification tools
It seems quite limited, since very few studies focused on this topic. The assessed reproducibility between three investigators is low for the Tunks classification and SCIP–IASP classification in a study on 29 subjects and 64 pain areas, with a moderate correlation between two types of classification (Kappa coefficient from 0.33 to 0.65), better between investigators for the SCIP–IASP classification (61 to 78% of agreement) than for the Tunks classification – 45 to 48% – . The Cardenas classification shows, according to a postal survey study on pain location and type, a good reproducibility between two investigators, with a Kappa coefficient at 0.68 for 41 subjects and 68 types of pain . Based on 179 pain complaints, 83% were correctly classified by a sample group of 39 experimented clinicians who evaluated the Bryce–Ragnarsson classification , with better answers regarding the level and type of injury (mean Kappa coefficient at 0.70, from 0.55 to 0.91).
The limits of these studies are connected to the methodology, because it is based on a classification established from items indicated on a questionnaire.
1.3.3
Evaluation
1.3.3.1
General framework
There is no specific tool for evaluating pain, either for measuring its intensity or regarding its nature and various characteristics in SCI patients. Most studies on pain in SCI patients found in the literature report the use of the Visual Analog Scale (VAS) or a basic numeric scale (NS) with 11 points (from 0 to 10) to evaluate the pain’s intensity but above all to measure the results of the implemented pain-relief therapeutics . Some studies use the Mac Gill Pain Questionnaire (MPQ) but none of them report a questionnaire specifically tailored to SCI patients and particularly to their neuropathic pain .
Recently, some studies focused on the impact chronic pain had on the quality of life of SCI patients , but without being specifically directed towards the neuropathic component of the pain.
Thus, the tools that are available and used for evaluating chronic neuropathic pain are not specifically tailored to SCI patients. The only tools that will be considered are the ones frequently reported in the literature in epidemiological or clinical studies, that are consensual and universally used by practitioners in charge of patients affected by chronic pain, and that were also regularly used in studies specifically focusing on SCI patients.
1.3.3.2
Evaluation tools
We should differentiate three types of tools: firstly, the tools geared for a global evaluation of chronic pain; secondly, those that are essential for evaluating any type of pain and thirdly, those that are specific to neuropathic pain.
1.3.3.2.1
Overall evaluation of chronic pain using validated tools
- •
Visual Analog Scale (VAS), Numeric Scale (NS), Verbal Rating Scale (VRS).
These various one-dimensional scales are designed to evaluate the global pain’s intensity or its improvement, by comparing the pain’s intensity reported by the patient at different times . However, they cannot be used to compare one patient to the next. Used by the patients to describe their pain, they are called self-assessment scales.
The VRS scale is made up of four or five ranked categories with a score from 0 to 4. The NS lets patients evaluate their pain’s intensity with a score going from 0 to 10 (or 100). The score 0 means “no pain” and the maximum score “extreme pain”. The VAS is a horizontal line, 100 mm in length, anchored by word descriptors at each end “no pain” to “very severe pain”. The patients mark on the line the point that they feel represents their perception of their current state. The VAS score is determined by measuring in millimeters from the left hand end of the line “no pain” to the point that the patient marks. These global scales are easy to use and can give a fast answer to the health care professional in order to evaluate how the patient responds to a pain-relief treatment or how the pain changes within a given specific framework. The NS does not require a specific support and is thus easy to use for pain measurements in daily life activities or during movements that can increase the pain.
- •
Mac Gill Pain Questionnaire (MPQ).
The MPQ consists primarily of three major classes of word descriptors: sensory, affective and evaluative, that are used by patients to describe their specific subjective pain experience, it also evaluated some semiological characteristics found in neuropathic pain . There is a shortened version that has been validated into the French language . The questionnaire consists of 102 words describing the patient’s subjective pain experience, these words were chosen based on the patients’ vocabulary used to describe their pain. The MPQ allows for a multidimensional, quantitative and qualitative pain assessment, the analysis brings in quantitative and qualitative nuances. The words are categorized into four groups and 20 subgroups. In each subgroup, the patients either choose a word or do not answer if no word can describe their pain. The words in each subgroup are ranked by increasing pain severity. Each subgroup is attributed a score: 0 if the patient does not answer, then 1,2, etc.The first group evaluates 10 items of the patients pain experience (example: numbness, scratching, tearing, squeezing). The second group evaluates the affective or emotional aspects of the pain experience: tension, fear, neurosensory reactions. The third group evaluates the cognitive aspect and describe the overall subjective intensity of the pain. The fourth group includes various word descriptors that did not fit into the previous groups. Many studies have validated the relevance of this tool in SCI patients .
- •
Questionnaire Douleur de Saint-Antoine (QDSA) is the shortened version of the MPQ validated in the French language .
This questionnaire has 60 word descriptors categorized into 17 subgroups including nine sensory groups, seven affective groups and one evaluative group. The patients pick the word descriptors and score them from 0 (not at all) to 4 (extremely). This self-assessment scale is quite simple and can refine the sensory and affective aspect of the pain’s intensity. Its limits are words incomprehension (sociocultural level) and being unable to proceed with frequent self-administrations of this scale.
- •
Brief Pain Inventory (BPI).
The BPI , with a validated version in the French Language ( questionnaire concis de la douleur ), evaluates the pain’s impact on the various daily life activities, using numeric 0 to 10 scales, with 0 being “no interference” and 10 being “interferes completely”, the BPI asks for ratings of the degree to which pain interferes with mood, walking and other physical activity, work, social activity, relations with others, and sleep. The mean of these scores can be used as a pain interference score. This is a pain-specific questionnaire that can be used in various circumstances.
- •
Evaluation of the pain-related psychological state.
The psychological evaluation of patients with chronic pain can be facilitated by using specific scales, especially for anxiety and depression. There are several tools available and the ones that are the most used for pain evaluation are the Hamilton Anxiety Scale, the Beck Depression Inventory (BDI), Montgomery and Asberg Depression Rating Scale (MADRS), Zung Self-Rating Depression Scale and the Hospital Anxiety and Depression Scale (HADS) useful for identifying anxiety and depression in patients .
- •
Quality of life questionnaires.
Quality of life questionnaires are very often used and highlight the impact that pain has on the patients’ quality of life, however these questionnaires are not specific to one particular type of pain or a neurological lesion . There are numerous questionnaires available, most often with good metrological characteristics (Sickness Impact Profile, General Health Questionnaire) .
1.3.3.2.2
Specific evaluation for neuropathic pain
- •
Neuropathic Pain Scale (NPS) not translated into French.
The self-administered multidimensional assessment tool has not been fully validated yet , and was the focus of an additional study to determine its sensitivity to change . This tool lacks specificity even though the discriminating and predictive values of the 10 words descriptors chosen by the authors were evaluated. However, some items that are specific to neuropathic pain (paresthesia, dysesthesia, allodynia to cold, paroxysmal pain) are missing. It is not very useful in daily clinical practice.
- •
Neuropathic Pain Symptom Inventory (NPSI).
It is a self-administered assessment questionnaire, designed in the French language ( Table 4 ), where the psychometric characteristics validated the discriminating nature and quantifying relevance of five different clinical aspects of neuropathic pain . The inter-user reproducibility was evaluated, as well as its sensitivity to change. The linguistic validation ensured that the word descriptors used for each item were simple yet specific enough. Finally, only the word descriptors selected in the factorial analysis were kept, with a 12-item final questionnaire identified by words descriptors for the pain that were location and time-specific for some of them. The severity rating scale consists of 11 points (Lickert scale: from 0 to 10) positioned on an horizontal line under each question. The temporal evaluation of the spontaneous continuous and paroxysmal pain is divided into five subgroups of increasing frequency. The total score is the sum of all the score on a total of 100. The subscores are for each of the five aspects of neuropathic pain: spontaneous superficial pain/spontaneous deep pain/paroxysmal pain/evoked pain/paresthesias and dysthesias. The semiological word descriptors are classified into five clinical aspects of neuropathic pain and validated as being representative of the various types of neuropathic pain. It is a reliable tool for an investigator faced with patients potentially affected by neuropathic pain: it is a refined, rigorous and validated extension of what has been done for years in the evaluation of patients with chronic pain. One important aspect is that the total numeric score is correlated to the VAS global pain score reported by the patient. Furthermore, the psychometric properties of the NPSI suggest that it might be used to characterize subgroups of neuropathic pain patients and verify whether they respond differentially to various pharmacological agents or other therapeutic interventions. The NPSI is an easy-to-use clinical description similar to the objective evaluation obtained by the quantified sensitivity study, thus allowing the physicians to not systematically perform this exam only available to some specialized teams.






