CHAPTER 10
Chemoneurolysis With Phenol and Alcohol: A “Dying Art” That Merits Revival
Lawrence J. Horn, Gurtej Singh, and Edward R. Dabrowski
For the three decades before the introduction of botulinum toxins (BoNTs) for the treatment of upper motor neuron syndrome (UMNS) and spasticity, physiatrists were trained in the use of phenol and, less commonly, alcohol for these purposes. In some situations, the technique to apply these agents was considered to be more laborious and required a modicum of greater skill than is the case with BoNTs. Given the relative facility of application of BoNT, its use among physiatrists and other specialists dramatically increased, whereas that of phenol declined. Hence, in the recent past, it was not unusual to refer to those of us using phenol (as well as BoNT) as practitioners of a “dying art,” particularly by those specialists who had never been trained in the application of phenol or alcohol.
The authors’ purpose in this chapter is to review the mechanism of action, techniques, proven benefits, and risks associated with phenol neurolysis along with a brief discussion of the use of alcohol. In addition, the authors provide a comparison between phenol and BoNT and suggest situations in which the practitioner may preferentially select one intervention over another. Given the limitations of BoNT (expense, the necessity of waiting 3 months between interventions, and the duration of action of BoNT), it is reasonable to include phenol and alcohol neurolysis, along with BoNT, in the armamentarium of the less-invasive approaches to the management of the UMNS.
CHEMICAL NEUROLYSIS
Nerve blocks involve the application of substances to a nerve that will interfere with conduction along the nerve on a temporary or permanent basis; local anesthetics, phenol, and alcohol are most frequently used. Chemical neurolysis involves the application of an agent that will damage a portion of a nerve, impeding conduction. Ultimately, these interventions are intended to treat spasticity, hypertonicity, primitive movement patterns, and possibly other aspects of the UMNS by interfering with the muscle stretch reflex arc. Nerve blocks exhibit their effect primarily through treatment of the efferent component of the arc, but the afferent loop can be involved as well. In essence, the result would be the conversion of a muscle affected by the UMNS to one with a partial lower motor neuron syndrome via partial denervation. Neurolysis has been used at every level of the peripheral nerve, from the spinal cord and roots to the motor end plate. The location of intervention will determine the completeness of the block and the number of muscles affected by the treatment.
Phenol and alcohol have been used effectively since the late 1950s. These substances have proven effectiveness in eliminating clonus, improving the range of motion (ROM) of joints affected by spastic contracture, reducing scissoring during ambulation, improving seating, improving gait, facilitating the use of orthotics, and ameliorating painful spasms or toe clawing (1–11). In the past, neurolysis has been used effectively to treat spastic external sphincters and reduce urinary retention (4). Several authors have described improved activation, strength, or speed in antagonists of blocked muscles. There is also evidence of a seemingly paradoxical improvement in the strength/control of partially blocked muscles themselves (2–5,8,11). Chemical neurolysis has a long history of proven effectiveness and benefits in the management of UMNS.
PHENOL
Phenol is carbolic acid, a derivative of benzene. At room temperature, it is soluble in water at concentrations less than 6.7%. It is also soluble in other commonly used vehicles such as glycerine and is available as colorless hygroscopic crystals. When oxidized, the crystals or phenol solution becomes pink. Phenol has local anesthetic properties at concentrations of 1% to 2%; it is bacteriostatic at 0.2% concentrations (in water) and bactericidal at 1.0%. In the past, phenol has been the active component in fungicidal skin preparations and was used for embalming by the ancient Egyptians. At the present time, it is a component of some over-the-counter throat lozenges. Phenol is considered a chemical, not a drug, by the Food and Drug Administration. As such, it is not technically “approved” for the treatment of spasticity.
When applied to or injected into any tissue at concentrations 5% or greater, phenol denatures protein causing tissue necrosis. This property accounts for both its effectiveness as a neurolytic agent and some of the associated potential side effects. Systemic doses of 8.5 g are considered lethal, principally from cardiovascular failure and severe central nervous system dysfunction including seizures. Doses of phenol injected for neurolysis are a tiny fraction of the lethal dose. Twenty milliliters of a 5% solution contains 1 g phenol. Phenol is easily absorbed through the skin (and dura, for those incorporating it as a component of prolotherapy). After injection, any systemically absorbed phenol is converted by the liver to phenyl compounds and excreted by the kidney as quinols. Chronic exposure can cause renal toxicity, rashes, or gastrointestinal problems.
Phenol Neurolysis: Histologic Changes
Initially, phenol was thought to selectively block small sensory fibers. However, the early electrophysiologic findings after application of phenol to peripheral nerves and nerve roots appear to be more relevant to its anesthetic and not its neurolytic properties (12–15). Subsequent histologic and electron microscopic examinations have demonstrated nonselective destruction of nerve fibers of all sizes (15,16). These findings may have implications for the techniques of application and may relate to injection-related complications.
Soon after phenol injection, inflammatory reactions occur followed by patchy areas of complete destruction of nerve fibers in roots and peripheral nerves (17). Burkel and McPhee (16) determined that if phenol is “dropped” onto a nerve, axons in the center of the nerve are spared, but if the chemical is injected into the nerve, all fibers are affected. Wallerian degeneration occurs at the site of injection, followed by subsequent regrowth of most axons. At 14 weeks, the injected/regenerated nerves appeared histologically normal, albeit with increased associated collagen and fibroblasts in the endoneurium. Lower concentrations of aqueous phenol (<1%) were more likely to cause localized demyelination without axon destruction (14–18).
In 1977, Halpern (19) reported his findings from evaluation of 144 samples from animals after intramuscular neurolysis with 1% to 7% phenol. Axons of all sizes were destroyed regardless of concentration. Neurogenic atrophy and collateral reinnervation were observed. Muscle recovery varied depending on the concentration of the aqueous phenol: 1% to 3% allowed for near-normal appearance of the affected muscle by 3 months, but specimens injected with 5% to 7% continued to show evidence of denervation. The volumes of aqueous phenol used for injection also correlated with the lesion size.
The clinical implications of the aforementioned histologic examinations may be summarized as follows:
1. Very low concentrations of phenol (<1%) may only produce local anesthetic effects or mild demyelination with temporary clinical effects.
2. Low concentrations (<3%) may produce a mixture of axonal destruction and demyelination with a possible reduction in the duration of neurolytic block, but with near-normal recovery of nerves and muscles.
3. Concentrations of 5% to 7% are more likely to leave residual evidence of denervation in nerves and muscles at 3 months, a longer duration of clinical effect, but with greater potential for some “permanence” to the block. Most current practitioners use concentrations of 4% or 5%.
4. When phenol is applied to nerves without penetrating them, it essentially affects what it contacts. Therefore, the practitioner may need to move the needle around the nerve and inject at more than one location in its circumference to reach specific fascicles or nerve bundles to achieve the desired clinical effect (bearing in mind that larger volumes will produce larger lesions in nerves and surrounding soft tissues).
5. The development of fibrous tissue in and around the nerve and adjacent soft tissue, coupled with collateral reinnervation and sprouting, may make a desired clinical outcome more challenging with subsequent injections, especially at the site of the original injection.
Technique of Injection for Phenol Neurolysis
Although some clinicians stimulated peripheral nerves and attempted to use electromyographic recording to isolated motor end plates (thus improving localization), this has proven to be a very laborious process and one that offers little advantage in precision when compared to careful clinical observation. The most commonly used technique employs the use of a nerve stimulator capable of delivering pulsed direct current as a square wave of 0.1- to 0.5-ms duration once or twice per second. The stimulator should have a rheostat to control the current flow. An ammeter facilitates precise localization. Generally, stimulators attached to electrodiagnostic equipment do not have ammeters, and “guessing” the milliampere flow is not a recommended technique. Localization with this equipment is akin to the use of early electrodiagnostic equipment, the chronaximeter. Essentially, motor points and nerves will respond to much lower amperage of current than surrounding muscles and soft tissue. Because the clinician wishes to preferentially inject proximal to the motor point or nerve, it is desirable to localize a site proximal to these structures, which is identified by obtaining a stimulation with less current. The current flow is between the stimulating electrode and a reference or pad; there is no ground. The needles used with the stimulator are the same as those used for BoNT injections: Teflon coated, except the bevel, of varying lengths dependent on site and patient morphology, and 22 to 27 gauge often related to the length of the needle. The primary author prefers to connect the needle to the syringe containing the phenol with a short length of intravenous tubing. This allows manipulation of the needle and maintenance of its position when withdrawing before injection and during the injection itself. If possible, a stimulator that allows for superficial, as well as percutaneous, stimulation is preferred. This will allow the clinician to localize nerves, angle of approach, and motor points before the introduction of the needle and facilitate more rapid percutaneous localization with less discomfort to the patient. Of course, superficial stimulation can only be employed for accessible nerves and muscles; deep-muscle motor points (eg, tibialis posterior) may only be reached percutaneously.
The patient should be prepped by having the entire limb being treated exposed. On occasion, special positioning techniques will need to be employed. If accessible nerves or muscles are to be treated, the use of a superficial stimulator to identify points along the course of the peripheral nerve to the motor point will help optimize localization. The current flow is typically between 5 and 30 mA for superficial stimulation. The clinician uses the superficial stimulator to identify the general location of the nerve or motor point by observing for the desired contraction of the muscle or muscle groups to be treated. The current is then reduced while observing for maximal contraction for finer localization (Figures 10.1 and 10.2). The point is then marked, and the skin at the site of injection is prepared with alcohol (or povidine and alcohol). The percutaneous electrode (needle) is then introduced. Most stimulators that allow for both percutaneous and superficial stimulation will require a modification of electrode feeds on the stimulator apparatus. A “search” is then undertaken, often in three dimensions, with the pulsed current typically between 1 and 5 mA. Once maximal desired contraction is obtained, the current is reduced and the localization proceeds as the clinician identifies the site of maximal contraction with the amperage less than 1 mA (optimally closer to 0.5 to 0.7 mA)—the lower the better for precision. This will indicate that the bevel of the needle is optimally positioned. If too much current is used when injection occurs, the “ball of current” at the bevel tip may stimulate nerve or motor points at some distance from the needle; higher volumes of phenol than should be necessary may be required to reach the target area. When the clinician feels that he or she has optimally localized the point for treatment electrically, the phenol should be slowly injected (Figures 10.3 and 10.4). Although as clinicians we are taught to withdraw before injection, this may be even more important with phenol because the potential for toxicity as a result of intravascular injection of the substance is very great. Once the phenol is injected, there should be an almost immediate reduction/cessation of the muscle twitch, with a continued reduction in response to electrical stimulation over 1 to 2 minutes. This occurs for two reasons: the initial local anesthetic effect of the phenol and the fact that the volume of the fluid may impede electrical conduction to some extent. The clinician also needs to consider that the bevel of the needle is directional; there may be a ball of current around the tip, but the injected phenol will flow out of the needle in one direction; therefore, if the clinician feels that he or she has accurately localized the point of injection electrically, yet with injection there is only a minimal reduction in contraction, he or she may find an improved effectiveness of injection by gently twirling the needle during slow administration of phenol. The total volume of phenol injected at one site typically ranges from 0.1 to 1 mL. The total volume of phenol administered to an adult in a given treatment session should not exceed 1 g. We limit our injections to 20 mL of 5% phenol for an adult but typically use less than 10 mL; unlike the case with botulinum, the patient may return in a few days for additional treatments if needed.
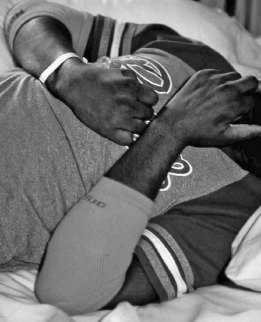
FIGURE 10.1 Early spastic contracture of the left elbow in a patient with traumatic brain injury.
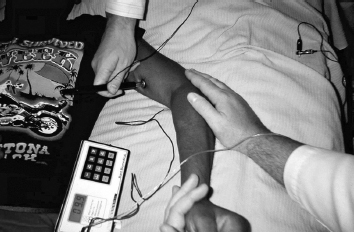
FIGURE 10.2 Superficial identification of left biceps motor point. Notice that the milliamp flow is 9.9.
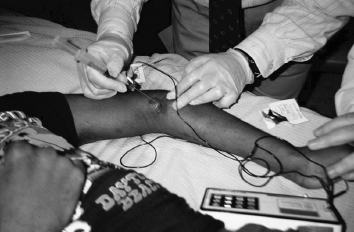
FIGURE 10.3 Percutaneous identification and neurolysis of left brachioradialis motor point.
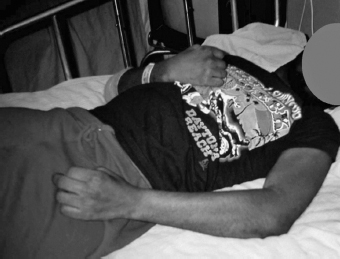
FIGURE 10.4 Result of phenol neurolysis of left biceps and brachioradialis motor points, as compared to untreated right upper limb.
Although the aforementioned process may sound laborious, normally it actually takes place within a minute or two; of course, the speed of the clinician improves with experience. Patient tolerance for the procedure varies considerably; patients may feel some discomfort from the electrical stimulation, from the needle search, and from some burning during the injection of phenol or alcohol. After the treatment session, we typically apply ice or cold packs to the areas of injection to help minimize discomfort from the needle searches. To expedite localization of motor points, various guides for the electromyographer (20) may be used. In addition, there are specific anatomic localization guides for phenol/alcohol neurolysis (21–23).
Site of Injection
Percutaneous Identification and Neurolysis of Left Brachioradialis Motor Point
As mentioned previously, the lower motor neuron may be, in theory, treated with phenol neurolysis at any point along its course from the spinal cord to the motor point of an individual muscle. If there is a single offending muscle, then one should localize that individual muscle through a motor point injection. However, in some cases, a single injection of a peripheral nerve may treat several muscles, contributing to a pattern of spasticity or primitive movement patterns (eg, tibial nerve injection in the popliteal space for equinovarus of the foot/ankle with associated toe clawing). Furthermore, given the relative immediacy of the action of phenol, the clinical effect may be titrated during the treatment session: Motor points may be injected, and if the effect is insufficient, the clinician may then move proximally to the motor branch or ultimately the peripheral nerve supplying the muscle. This also allows for the patient to “try the block on for size” as to his or her satisfaction with the result, particularly for gait disorders or orthotic fit, with the potential for the clinician to go back and do additional treatments until the patient is satisfied with the result. Ultimately, the choice of site (motor point, motor nerve, or mixed sensory/motor nerve) will be dictated by the desired clinical effect (including number of muscles being treated in the distribution of a peripheral nerve), the tolerance of the patient for the needle search, the risks of the needle search at different sites, and the risks for other complications such as dysesthesias.
Lumbar and sacral nerve blocks. This procedure is no longer a common technique of treatment. It requires conscious sedation and fluoroscopy to provide optimal localization for the needle. Risks include accidental intrathecal injection and damage to the entire cauda equina with associated sexual, bowel, and bladder dysfunction. Most often, this procedure was undertaken to treat hip flexors. Alternative treatment approaches with BoNTs have all but eliminated this particular intervention technique.
Mixed motor/sensory peripheral nerve blocks. There are many advantages to this procedure compared with motor point blocks. First, there is a block of sensory fibers, cutaneous and from the muscles themselves, both of which may contribute to the reduction of spasticity. Second, it is often possible to identify bundles within the nerve and preferentially block them to target specific muscles with one injection site. Third, most clinicians believe that sensorimotor peripheral nerve blocks produce a more thorough block of longer duration, although this belief does not have more than a teleological basis for support in that it has not been systematically studied. Certainly, a more proximal block will require a longer period of axonal regeneration insofar as this contributes to the recurrence of spasticity. Fourth, this technique is often easier and faster than more peripheral motor branch/point blocks in achieving a desired clinical effect.
The major risk of this type of injection is related to one of its theoretical advantages: the block of sensory (and possibly autonomic) nerve fibers. This can lead to temporary loss of sensation and to the potentially much more bothersome or disabling nerve pain in the distribution of the injected nerve. Identification and management of these problems are discussed later in the chapter. In addition, the immediate effect of the block can be quite profound and potentially lead to “overcorrection” or overstretching injury if the patient is not properly counseled.
Motor nerve/motor point blocks. There are many points along the peripheral arborization of motor nerves where these blocks can occur. In some cases, the motor branch to a specific muscle can be identified very close to its departure from the mixed sensory/motor nerve from which it originates. More commonly, the motor fascicle is identified near the muscle it innervates. Motor points are the place where this motor branch enters a particular muscle or where there is a cluster of motor end plates. There are usually several motor points for a given muscle. Therefore, as one attempts neurolysis more peripherally along the motor nerve, more injections are necessary. The patient has to endure more needlesticks, and the clinician may become more fatigued over time. The advantage of injecting motor points or motor branches is that they do not contain any cutaneous afferents, and hence, the risk of dysesthesia is greatly reduced compared to mixed sensory motor nerve blocks. Furthermore, it allows the ability to titrate the clinical effect of the block alluded to previously. Certain peripheral nerves (most notably the obturator nerve) contain little to no sensory components and therefore do not have associated dysesthesias with whole nerve blocks.
Open neurolysis. A final technique, also not commonly used, is the open, surgical identification of peripheral and motor nerves followed by the application of phenol. Obviously, this allows for direct visualization of the nerve to be treated and may allow for safer proximal treatment. However, this procedure incorporates the inherent risks of anesthesia and the additional recovery from surgery, along with added costs.
Duration of Action of Phenol Neurolysis
There is an extraordinarily wide variability in the literature regarding the duration of the effect of phenol. Again, from a teleological perspective, one would assume that the duration of action would be longest for root or peripheral nerve blocks when compared to intramuscular or motor point blocks; it takes longer for axons to reinnervate target muscles the more proximal the injection. However, this is not clearly demonstrated in the literature (Table 10.1). Peripheral nerve blocks appear to last from 10 days to as many as 28 months (10). Paravertebral blocks range between 1.5 and 10 months (24) and intramuscular blocks between 1 and 36 months (25,26). Specific motor point or end-plate blocks were reported by DeLateur (8) to last 3 to 6 months and open nerve blocks 6 months to more than 1 year.
TABLE 10.1
EXPECTED (ESTIMATED OR PROJECTED) DURATION OF PHENOL NEUROLYSIS | |
Anatomic Location | Duration |
Peripheral nerve block | 10 days–28 months |
Intramuscular block | 4 weeks–36 months |
Paravertebral block | 6 weeks–10 months |
Motor point or end-plate block | 3 months–6 months |
Open nerve block | 6 months–12 months |
The variability in the literature may be explained by several factors. The percent (ranging between 2% and 5%) of aqueous phenol as well as volume (not consistently reported) could account for some discrepancies. The measurement tools used for spasticity and return of spasticity were also quite different and at times extremely subjective. Although it is assumed that all clinicians in the studies were experienced with phenol neurolysis, the techniques used may also influence the outcome/duration. Furthermore, it is not entirely clear from the peripheral nerve studies whether there was evidence for blockage of sensory afferent fibers that might contribute to spasticity.
Other factors might contribute to improving the duration of outcome related to postblock interventions. If the injected muscle and associated soft tissue are effectively stretched after the block, this may prolong the clinical effectiveness. If the antagonist is strengthened either by exercise or by electrical stimulation, it may, in theory, produce a clinical improvement in the duration of efficacy through inhibition of the injected muscle.
Repetition of blocks may also improve the duration of effectiveness with the subsequent injections (27). However, most studies (and clinical experiences) that evaluated repetitive blocks seem to indicate that it is somewhat more difficult to perform the subsequent blocks (25). Both the difficulty in achieving the block and the improved duration of effect may be explained by fibrous tissue and sprouting that make localization more difficult but may also impede regrowth of axons.
Side Effects and Complications of Phenol Neurolysis
Side effects of phenol neurolysis may be related to the simple act of placing a needle in the soft tissue (bleeding, compartment syndrome, pain, infection); the effects of the block (overcorrection, strain or sprain from overstretching, temporary loss of useful motor function, atrophy); and specific side effects from phenol (temporary sensory loss, dysesthesias, tender nodules in soft tissue). Venous thromboembolism has been rumored to occur, but the evidence to support that this is an effect of phenol is weak to nonexistent. Patients who were purported to have experienced this problem were few, and evaluations for hypercoagulable states or absence of venous thrombosis before injection were not done. Macek (28) reported (second-hand) on two cases with purported venous thrombosis related to phenol neurolysis. In neither case was there a specific discussion of the location of the deep vein thrombosis or effectiveness of the blocks. Technologies were limited in the 1980s, but there was no discussion of coagulation disorders. Theoretically, a poor technique with intravascular or perivascular injection could cause scar tissue damage or occlude venous structures. A particularly effective block might render the “pumping action” of spastic muscle ineffectual, contributing to venous stasis (22). In a similar vein, if the patient was uncomfortable after the block or took a long car or airplane trip, inactivity could lead to stasis as well. Awad (22) has articulated the opinion that when deep vein thrombosis occurs, it is usually in the calf and may be related to the “repeated indiscriminate needle probing and/or the injection of large quantities of phenol solution by the novice.”
Side effects from needling. Local bruising, pain, and swelling may occur, as well as infections, if an aseptic technique is not utilized. Aspiration before injection is an obvious precaution. A potential complication relates to bleeding in deeper muscles and the risk of a compartment syndrome. Many patients with stroke are on antiplatelet therapy and may bleed more easily; special care in minimizing needle exploration should be exercised under these circumstances. It is also advisable to stop coumadin or therapeutic anticoagulation in a sufficient time frame before injection to avoid this complication.
Side effects from effective blocks. These include the possibility of atrophy, sprains, and overcorrection, as well the potential loss of useful motor function. There may also be a temporary loss of cutaneous sensation. In terms of atrophy, caution should be observed in exercising the involved musculature to fatigue during the period the block is most “active.” Subsequently, after the block has begun to wear off, electrical stimulation and exercise (and the return of spasticity) are usually sufficient to correct what is largely a cosmetic problem.
Peripheral nerve blocks may be very profound in their effect, with clinical changes in several muscles involved in a particular pattern of movement. Most patients will have become accustomed to their spasticity and will have learned compensatory mechanisms for functional activities. The best example would be the use of a tibial nerve block in the popliteal space to treat equinovarus of the foot/ankle as well as toe clawing. In comparison to motor point blocks or BoNT, there may be a much more complete and immediate abolition of spasticity and possibly motor function in the gastrocnemius, soleus, tibialis posterior, and long and short toe flexors. As a result, the patient does not have the opportunity to acclimate to changes in their spasticity as might be the case with botulinum. Caution should be observed immediately after the block if the patient is accustomed to ambulating. Overstretching of the involved parts should be avoided as well. In this example, there may also be loss of the intrinsic muscle functions of the foot, resulting in sprain from poor dynamic support of the plantar arch; this may be prevented by the use of a foot orthotic or modification of the sole plate of an ankle-foot orthotic. At times, there is overactivity in antagonist muscles that result in “overcorrection” as may be seen in surgical interventions to lengthen or move tendons. In this example, if the tibialis anterior were a powerful contributor to the varus posturing of the foot and ankle, one may see an associated “calcaneal” deformity, at least dynamically, of the foot/ankle complex after tibial nerve block. Appropriate intervention would include motor point blocks or BoNT injection to the tibialis anterior or peroneal nerve block. Most of the problems of overcorrection can be avoided by not being overzealous with injections and titrating the amount and site of injection to the patient’s need.
In evaluating patients before neurolysis, the clinician must be cognizant of the risk of loss of useful motor function, even if it is dominated by spasticity and is under poor volitional control. Gait (7,9) or transfers may be compromised.
Side effects specific to phenol. These side effects are in part related to the location of the block. Although no longer commonly done, there is the possibility of dural transfer of phenol when done at the level of the nerve roots. The affinity of phenol to cross dura, without the direct penetration thereof, has been confirmed in animal experiments (15). In humans, this may account for case reports of spinal cord injury when the injection was done close to the dura (29,30). Intrathecal injection of phenol has also been associated with thrombosis of the posterior spinal artery (31).
Dysesthesias. The most worrisome side effect of phenol neurolysis in mixed sensory/motor nerves is that of dysesthetic pain, which occurs in the sensory distribution of the nerve. The pain is of the classical neuropathic type: burning, tingling, and electrical feelings, exacerbated by light touch. Onset is not immediate, but delayed by a few days to 2 weeks. The incidence of dysesthetic pain varies widely in the literature (0%–32%) and is affected by several variables. There are no reports of dysesthesia with obturator nerve blocks; so in reviewing a series of patients, it is important to identify the percentage of blocks done to this nerve. The skill of the clinician may be relevant, but there is no clear association with the concentration or volume of phenol. The duration of the discomfort is typically several weeks (21), but may last a few days to as much as a year. Kolaski et al (32) reported that the use of phenol and botulinum in combination produced a higher rate of complications than botulinum alone, although patients receiving the combined treatment were more likely to have greater severity of spasticity. Most of the complications were related to local injection symptoms; the rate of dysesthesias was only 0.4%. However, the mixed sensorimotor nerves injected were the obturator (no reports of dysesthesia) and the musculocutaneous.
The duration and severity may be ameliorated by pharmacologic and other interventions. Classically, the recommended intervention is to repeat the block with the thought that the original intervention was incomplete (10), but it is often difficult to convince patients to repeat the procedure that caused their discomfort in the first place. Modalities for management include compression garments or wraps. Local anesthetic patches may be effective. The primary author would suggest a fairly aggressive pharmacologic approach if it is clear that the pain is neuropathic and not due to overuse or mechanical factors. This includes the use of anti-inflammatories (medrol dose-pack followed by nonsteroidal anti-inflammatories, if symptoms persist); anticonvulsants (pregabalin, gabapentin, carbamazepine); and antidepressants (duloxetine, amitriptyline), and this should be considered in combination and gradually weaned. Finally, an underrecognized complication may relate to the block of autonomic fibers traveling with the sensorimotor nerve. This may result in redness or other discoloration of the skin supplied by said fibers, presumably due to loss of neurogenic vascular control in a paretic limb. There may be associated edema, and the treatment is reassurance and compressive garments.
Local inflammatory responses. Motor point blocks often involve several injections in a given muscle. As a result, patients may have transient pain or swelling related to both repetitive needling and the inflammatory effects of phenol. This is particularly true in the calf. Tender nodules may develop and may reflect the local damage to muscles with an associated inflammatory reaction described by Halpern (19) in animal studies. These reactions began a few days after injection and began to resolve after 2 weeks. Glenn (21) recommends that the application of icing immediately after the procedure and anti-inflammatory medications can prevent or ameliorate these temporary symptoms.
ALCOHOL
In addition to phenol, there has been both clinical and literature support for the use of ethyl alcohol (alcohol) in the chemoneurolysis of peripheral nerves as a part of a treatment plan for spasticity (33–37). Although the volume of literature support is limited as compared to phenol and now botulium toxin, there does remain strong efficacy for its use in both achieving desired outcomes (33–37) as well as limiting undesired side effects (34–39).
Jang et al (34) treated spastic hemiplegia of the ankle using 50% ethyl alcohol in water. They identified the motor branches of the tibial nerve responsible for both the medial and lateral heads of the gastrocnemius muscle. Postinjection and then at 6-month follow-up, there remained a statistically significant decrease in the Modified Ashworth Scale scores, clonus scores, and passive ROM. Although these three measurement tools are scientific in nature, possibly the addition of gait analysis might have further provided an added clinical value to their overall outcomes.
Viel et al (37) used 65% ethyl alcohol for 27 obturator nerve blocks. Radiographic films and stimulator guidance were used to achieve 100% localization. Using their own scales for muscle spasticity, triple flexion, gait, and hygiene, there were significant statistical data to show improvement at the 1-, 2-, and 4-month follow-up. An interesting aspect to their research was the use of cost analysis. They found a total cost of 10.67 euros for the procedure (8.23 euros for the needle and 2.44 euros for the alcohol solution). The cost for a 4-month supply of dantrolene sodium and oral baclofen was between 500 and 1,000 euros and between 170 and 134 euros, respectively. One significant cost that was not included in this study was for the use of a nonoperative procedure room and conscious sedation or generalized anesthesia used during the ethyl alcohol injection.
Kong and Chua (36) used 100% ethyl alcohol in the treatment of 13 patients with hip adductor spasticity. As with the study of Jang et al, they also found statistical improvement at 6 months in a decreased Modified Ashworth Scale score and an increased passive ROM. Gait analysis was performed in this study, but for only the three patients who could ambulate. Even postinjection there was a continued use of assistive devices; however, both patients and authors agreed that there was an improvement in overall balance, decreased scissoring, and increased gait speed. Further studies with a larger ambulatory population may certainly help show the long-term utility and retained functional outcome with the use of ethyl alcohol as an effective chemoneurolytic in patients with spastic gait.
In the early 1960s, Hariga et al (40) and Tardieu et al (41,42) proposed injection of a local chemical neurolytic agent directly into muscles. They injected large quantities of 45% alcohol into the limb muscles, at the motor point in children with cerebral palsy. It was reported that spasticity was reduced in most cases, without reducing or affecting voluntary strength. They further reported a duration effect from 6 to 12 months and occasionally as long as 2 to 3 years (40–43). Similar findings were noted by Cockin et al (44) in the United Kingdom in 1976. He injected 45% alcohol into similar muscles, yielding a reduction in spasticity without inducing profound weakness (44).
Other investigators evaluated the Tardieu technique (6,44–47), which led to modification of the procedure (6,45,46). O’Hanlan et al (6) used the same dilution of alcohol but did not try to target the motor nerves specifically. They injected 45% alcohol in large volumes of 10 to 40 mL into multiple locations within the target muscles of spastic patients. O’Hanlan et al observed significant reduction in spasticity without the loss of motor power in the 10 patients treated. Sensation was also reported to remain intact. They also reported in the Virginia Medical Monthly a case of vascular phlebitis when a “poor” so-called state store preparation of alcohol was used. Carpenter (45) and Carpenter and Seitz (46) proposed the concept of “intramuscular” alcohol using 40% to 50% alcohol. This procedure was performed under general anesthesia due to the significant pain caused during the injections. The gastrocnemius demonstrated the best response of those muscles injected. The muscles were divided into quadrants, and each quadrant was injected with 2 to 6 mL of alcohol. Equinus gait was eliminated in 128 of 130 children injected. The duration of the effect was shorter than that reported by Tardieu et al, with the equinus gait returning 7 to 20 days after injection. Overall, the injections lasted 1 to 6 weeks. Muscle biopsies were also performed 4 to 6 weeks after the procedures, and in four to six patients revealed a round cell infiltrate without fibrosis.
Intramuscular alcohol has been used to achieve relief of spasticity for a slightly longer duration and is less time consuming than for motor point blocks (46,48). In addition, the procedure’s duration of effect could be useful for diagnostic purposes when a longer period of evaluation is necessary than can be provided by a local anesthetic agent or for therapeutic purposes when longer-lasting procedures are unnecessary or undesirable. Another advantage of this procedure is that because precise localization of nerves is not necessary, it can be performed quickly in situations in which speed is a consideration, for example, in agitated and combative patients and in children (49).
There have been many questions as to the optimal concentration of ethyl alcohol that is to be used in the chemoneurolysis of peripheral nerves. In a classic study of animals, May (50) described how the use of 100% ethyl alcohol causes degeneration and fibrosis with partial regeneration of neurons. It also attempts to link how more dilute solutions do not show an equal correspondence to neuronal destruction and that less concentrated injections may be unpredictable in their overall outcome. Most studies indicate that the concentration of alcohol used is dependent on muscle mass and weight of the patient.
The mechanism of action of alcohol is as a nonselective denaturization of proteins that affect axons, myoneural junctions, muscle fibers, and the interstitial tissue. This may lead to retrograde Wallarian degeneration of the nerve fibers (38,39). Biopsies from muscles that have undergone intramuscular washing have shown necrosis and inflammatory cells (46).
There is no clear correlation of alcohol concentration with adverse side effects. Most studies have shown limited adverse outcomes with only one to two patients in each study complaining of local site injection pain or sensory dysesthesias, which lasts for only a few weeks and seem to respond well to select antidepressants (amitriptyline) or antiseizure (carbamazepine) medications (34,38). Theoretical risks are similar to those seen with the use of phenol and include seizures, central nervous system depression, and cardiovascular collapse (36). A complication unique to the use of ethyl alcohol is inebriation. One study of adults (36) did encounter this side effect but stated that it resolved spontaneously. Critics to the use of ethyl alcohol could theoretically consider its use in the pediatric population unwarranted; because of this, it is our opinion that most children undergoing this procedure are chaperoned by their legal guardians who can help to monitor such a reaction, and we would also advocate for limiting its use in those patients, children or adults, who have any form of liver damage that might inhibit their ability to process its systemic effects.
COMPARISON OF PHENOL NEUROLYSIS AND BoNT
There are several potential benefits of phenol over BoNT. Phenol has an immediate onset of action, is much less expensive, and can be titrated in a single visit to optimize dosing/clinical effect. BoNT dosing may be adjusted but cannot be administered more frequently than every 3 months. Phenol may be repeated in a few days. Indeed, if the patient or clinician is dissatisfied with the results of a BoNT injection (once it has reached full therapeutic effect), “touch-up” blocks could be done with phenol. Phenol can be given anywhere along the motor nerve, from motor point blocks (most akin to BoNT effects) to nerve roots. The duration of action of peripheral nerves or motor nerve blocks appears to be longer than those with motor point blocks or BoNT. Furthermore, it may be feasible to inject a single point on a peripheral nerve and treat several muscles simultaneously (tibial nerve block), sparing the patient multiple injections and often getting a more effective block than with BoNT.
On the other hand, there are potential risks to phenol as compared to BoNT. The immediacy of effect, particularly from robust peripheral nerve blocks, may require adjustment by the patient and may transiently interfere with gait or transfers; the more gradual onset of effect from BoNT injection is far less likely to cause this problem. Of course, the major concern from phenol neurolysis compared to BoNT is the potential for sensory disorders. As mentioned earlier, the exact incidence is not clearly known and varies depending on which sensorimotor nerve is injected. Overall, the incidence appears to be less than 10%; the hyperalgesia/dysesthesia phenomenon is also treatable and typically does not last very long with treatment—of course, the presence of this complication may put a bit of a strain on the patient–physician relationship for its duration. Phenol may also produce scars or fibrous depositions in soft tissue. Some of these may be uncomfortable and, more rarely, may affect subsequent surgical releases or other interventions.
Given the wide disparity in cost between phenol and BoNT, it is surprising that there are only a very few studies directly comparing these agents. The studies that do exist have serious flaws, and it is difficult to generalize from them. In 1998, Kirazli et al (51) reported on what was the “first trial” to directly compare BoNT and phenol for spastic foot after a stroke. Ten patients received 400 U of BoNT, acknowledged by the authors to be a relatively high dose, distributed as 100 U in each of the following muscles under electromyographic guidance: the soleus, medial gastrocnemius, lateral gastrocnemius, and tibialis posterior. Ten patients received 3 mL of 5% phenol injected as a tibial nerve block with percutaneous stimulation, “the primary target being fibers to the gastrocnemius and soleus muscles and the secondary target, the tibialis posterior.” The authors were allegedly attempting to do the block just distal to the bifurcation of the sciatic nerve, 7 cm above the popliteal crease. Yet, they also state that the phenol procedure took 1 to 2 hours, and the BoNT procedure only 15 to 30 minutes. Typically, a phenol block of the tibial nerve should only take 10 minutes.
Follow-up was done using the Ashworth Scale for dorsiflexion and eversion, clonus duration, improvement in active and passive ROM, Brace Wearing Scale, and an ambulation score. This follow-up occurred at 2, 4, 8, and 12 weeks. The authors concluded that the eversion Ashworth score improved in the botulinum group but not in the phenol group, and, overall, BoNT was more effective at weeks 2 and 4 but that there were no statistically significant differences at weeks 8 and 12. This would contradict the clinical experience that a tibial block would be of longer duration than motor point blocks or botulinum. Furthermore, 2 (20%) of 10 patients developed peroneal nerve palsy and 30% developed dysesthesias lasting 2 to 4 weeks in spite of the authors’ seemingly laborious efforts to accurately localize their phenol injections; these complications impacted the results in, at the very least, the Ashworth for eversion and walking capacity that favored BoNT in the first 2 to 4 weeks after injection. It is not clear whether the two patients with inadvertent peroneal palsies also contributed to the 30% of patients who developed dysesthesias. For an experienced clinician, it is not surprising that peroneal palsies developed in 20% of 10 patients and 30% developed dysesthesias given the “localization” of the tibial nerve 7 cm above the popliteal crease. This site is far more rostal than the one used by clinicians with experience performing phenol tibial nerve blocks. Typically, the nerve is identified at the popliteal crease, although additional blocks of the large motor branches to the gastrocnemius bellies may have to be identified just medial and lateral to the nerve at this level or just distally as they enter the bellies of the muscle. Glenn (21) comments on this potential flaw in technique: “When blocking at the apex of the popliteal fossa, the practitioner should be aware of the close proximity of the common peroneal nerve to the tibial nerve and the origin of the sural nerve at this level.” Therefore, although this study purports to show a relative advantage of large doses of BoNT for the spastic equinovarus foot compared to phenol, given the small number of patients, the findings and data are severely compromised by the technical inadequacies of the phenol blocks. From a practical perspective, hemiplegic patients may require treatment of additional muscles in other limbs or for spastic toe flexors or striatal toe associated with spastic equinovarus. The use of such large doses of BoNT for the targeted muscles in this study would also have a significant impact on the total dose recommended for the first or subsequent injection; less BoNT would be available to treat other limbs or even muscles involved in the same primitive movement pattern.
A recent retrospective study evaluated 336 children and 764 treatments comparing phenol and BoNT to BoNT alone for complications (32). Phenol use was confined to the obturator nerve and musculocutaneous nerve blocks. Complications were divided into intraoperative systemic, local, and functional. Patients injected with the combined agents were more likely to have severe impairments with longer treatment sessions and spastic tetraplegia. Systemic complications were more common in the combined group (7.6% vs 2.5%) but were of short duration (3–7 days) and could not be attributed to an effect of phenol versus BoNT by the authors (eg, constipation). Two patients (0.4%) did experience cardiac arrhythmias, which resolved with observation. One of these had mitral valve prolapse, which predisposes to arrhythmia. The authors commented about a previous study with a 19% rate of arrhythmia with phenol, but this study involved intramuscular injection and halothane anesthesia. Local symptoms were more common in the combined group (4.5% vs 1.5%), which included bruising, swelling, and tenderness, but these were not specifically ascribed to the sites of phenol injection, and again, the combined population was subjected to more extensive needling. Dysesthesias were rare (0.4%) and occurred only in the distribution of the musculocutaneous nerve. This is a low incidence compared to other studies and reflects the selection of mixed sensorimotor nerves with little or no significant sensory component. Overall, this study demonstrated the acceptably low risk of combining phenol neurolysis and BoNT injections, especially when treating complex patients where the total safe dose of BoNT limits the global clinical effect desired by treatment.
Hypothetical Cost Comparisons
Obturator nerve blocks versus BoNT for adducted lower extremities. To optimally treat scissoring gait or severe adductor tone for hygiene, 150 to 200 U of botulinum type A (BoNT-A) would be required at a cost of $507.42 × 2 = $1,014.84 versus 5 mL of 5% phenol ($7.00).
Musculocutaneous nerve block versus BoNT for flexed elbow. Two hundred units of botulinum A for biceps, brachioradialis, and brachialis at a cost of $507.42 × 2.5 = $1,268.55 or 3 mL of 5% phenol with low risk for dysesthesias.
Tibial nerve block with dysesthesias. Comparative cost analysis case scenario: A 48-year-old man with a traumatic brain injury presents to your clinic 6 months postinjury. He has completed both acute inpatient rehabilitation and an outpatient program. A spastic hemiparesis causing plantar-flexion contracture remains as a major barrier in his ability to progress from an assist level to a modified-independent level with activities of daily living (ADLs) and ambulation. Traditional oral antispasticity agents have either failed to resolve his contracture or caused excessive sedation. He is now unable to don an ankle-foot orthosis, even with the assistance of family.
TABLE 10.2
COST COMPARISON OF PHENOL TIBIAL NEUROLYSIS AND BOTULINUM FOR EQUINOVARUS OF THE FOOT/ANKLE | ||
Substance Used | Without Complication ($) | Treatment for Neuritis With Steroid Dose Pack and Pregabalin ($) ×3 Months |
BoNT-A | 2,029.68 | 2,029.68 |
(400 U) Phenol | 7.85 | 594.31 |
Interventional options are as follows:
• Phenol chemodenervation of the tibial nerve at a point in the popliteal fossa. Five milliliters of prepared 5% phenol in sterile water.
• BONT treatment to the medial and lateral gastrocnemius muscles, soleus muscle, and posterior tibialis muscle. One hundred units of botox per muscle group for a total of 400 U.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






