CHAPTER 8 Central convergence of viscerosomatic inputs from spinal and vagal sources
Introduction
The traditional view of the nervous system treats cutaneous structures and the internal visceral organs as separate entities. The skin forms a barrier with the external environment and provides precise information (e.g., mechanical intensity, temperature, spatial dimensions) that is conveyed to higher centers in the brain for processing via a number of well-defined pathways. Sensory afferent information conveyed centrally from the urogenital tract, upper and lower gastrointestinal tracts, and other viscera is involved in the regulation of internal organs and organ reflexes via sympathetic and parasympathetic efferent outputs. Specific sensations, such as pleasure, hunger/satiety, urges related to the need to urinate/defecate, and visceral pain, also involve higher-order processing. It is well known, however, that cutaneous and visceral systems do not function independently. For example, different types of somatic manipulation (such as different forms of manual therapy, acupuncture) produce precise changes in a variety of visceral systems (Berkley 1993). Integration in the CNS therefore includes not only the processing of information for the coordination of multiple organ systems (viscerovisceral interactions), for example, but also for the processing of inputs from both somatic territories and internal organs (viscerosomatic interactions).
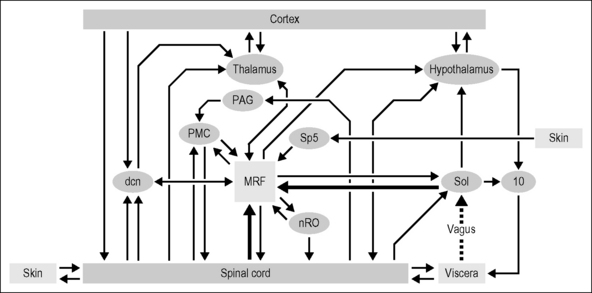
Central convergence of viscerosomatic inputs is found beginning at all three main ports of entry into the CNS: spinal gray matter, dorsal column nuclei, and solitary nucleus. These regions can influence each other through direct interconnections as well as indirectly via overlapping target regions across the entire neuraxis (Fig. 8.1). The interactions and contributions of these various ports of entry for affective/motivational systems and sensation, from pleasure to pain, are not well understood. One functional role associated with viscerosomatic convergence, for example, is referred pain of visceral origin, whereby convergence may be an efficient means through which visceral nociceptive information utilizes ascending somatic nociceptive systems. In this chapter, central convergence of viscerosomatic inputs will be reviewed at these three main ports of entry into the CNS, as well as within three of the major central relay areas (medullary reticular formation, thalamus, hypothalamus). The contribution of spinal versus vagal sources of input to the CNS for pelvic visceral sensations/functions is discussed within the context of our ongoing studies on spinal cord injury.
Viscerosomatic inputs to the CNS: three ports of entry
Spinal processing
The lumbosacral segments (L5–S1 in the rat) of the spinal cord in males contain a center for erection and the expulsive part of ejaculation. Numerous studies have been carried out to study the various elements of the spinal neural circuitry mediating these functions. For example, erection and ejaculation have been shown to be mediated by preganglionic parasympathetic motor axons in the pelvic nerve and somatic motor axons in the pudendal nerve supplying the striated perineal muscles of the pelvic floor (Coolen et al. 2004, de Groat & Booth 1993, Giuliano & Rampin 2004, McKenna 1998, Steers 2000). Animal experiments in which electrical stimulation is applied to the nerve that supplies the penis, the dorsal nerve of the penis (DNP), reveals expression of a neural activity marker (Fos protein) in the dorsal horn, dorsal gray commissure and sacral parasympathetic nucleus (Rampin et al. 1997). Individual spinal cord interneurons in the dorsal horn and intermediate zone of primarily L6–S1 that receive input from DNP afferents have been studied in vivo with electrophysiological recordings (Johnson 1989). All penile interneurons exhibit receptive fields on the penis that are significantly larger than the receptive fields for single primary afferent neurons, thereby demonstrating a central convergence of penile sensory input. In addition, most penile interneurons have receptive fields on both sides of the body, and their electrical characteristics strongly suggest a monosynaptic input from both ipsilateral and contralateral DNP fibers (Johnson 1989). There is also an extensive representation from the distal glans (‘cup’) region in these spinal cord interneurons. DNP afferents produce bilateral (crossed and uncrossed) reflex facilitation of pudendal motoneurons located in L5–L6.
In addition to the lumbosacral region of the spinal cord in male rats, a lumbar (L3–L4) reflex region for ejaculation has been identified that depends on input from afferent systems releasing substance P (Truitt & Coolen 2002). These lumbar neurons are located lateral to the central canal in lamina X and in the medial portion of lamina VII, and have projections to neurons involved in the emission as well as the expulsion phase of ejaculation.
In females, organ-specific and organ-characteristic information is conveyed in a rostrocaudal topographic array to the caudal spinal cord (Berkley & Hubscher 1995b, Berkley et al. 1993c). In vivo electrophysiological recordings as well as neuroanatomical tracing studies indicate that there is an extensive system of neurons in thoracolumbar and lumbosacral spinal cord that receive female reproductive organ inputs (Berkley et al. 1993b, Lee & Erskine 1996, 2000). The cervix, for example, which is innervated by both the hypogastric and the pelvic nerves, has inputs to dorsal horn neurons at both levels, although the neurons are concentrated ventrally in the dorsal horn at T13–L1 and throughout the dorsal horn at L4–L5 and L6–S1 segments (Berkley et al. 1993b). Cervix-responsive dorsal horn neurons in both regions receive convergent inputs from other pelvic organs such as the colon (51%) as well as cutaneous regions, although the receptive fields tend to be larger at T13–L1 and more confined to the perineum for the neurons at L6–S2 located in the dorsal part of the dorsal horn (Berkley et al. 1993b). Many cervix-responsive neurons at L6–S2, for example, respond to uterine distension by being inhibited. This uterine input originates from distant roots (uterus innervated by the hypogastric nerve), as shown in experiments where T13–L2 roots were sectioned bilaterally (Wall et al. 1993). How these interactions sculpt the actions of these neurons for various aspects of reproduction is unclear.
A somatic region that is consistently seen to converge with neurons having inputs originating from the female reproductive tract, particularly at the T13–L1 region but also in the deep dorsal horn at the L6–S2 region, is the hind paws (Berkley et al. 1993b). This is true throughout the neuraxis (see summary of studies on Relay Nuclei section below) as well as for penile-responsive neurons in male rats. The stimulus is deep pressure (squeezing) of the toes with a moderate (presumably noxious) force. Thus, the neural circuitry is such that stimuli or pathology affecting the pelvic organs can modulate the processing of inputs from the feet, and vice versa.
The dorsal column nuclei
The dorsal column nuclei (gracile – lower body; cuneate – upper body) is a region traditionally designated as receiving somatotopically organized input related to active touch and kinesthesia. Neurons in the gracile nucleus (Gr), however, have also been shown to receive afferent input from the external genitalia, internal reproductive organs, colon, pancreas, and kidney (Al-Chaer et al. 1996a, Berkley & Hubscher 1995a, Bradshaw & Berkley 2000, Cothron et al. 2008, Rong et al. 2004, Simon & Schramm 1984, Wang & Westlund 2001). Thus, in addition to the spinal cord, the dorsal column nuclei in the caudal brain stem are another port of entry into the CNS where somatic and visceral interactions and modulation of inputs can occur.
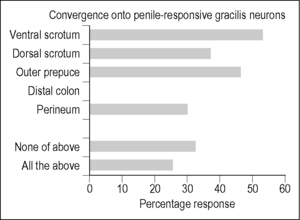
Evidence for such convergence and thus the potential for interactions once again comes from experimental studies in animal models. In male rats, several neuroanatomic tracing studies have shown that injection of a tracer such as fluorogold or horseradish peroxidase (HRP) into the pelvic or pudendal nerve labels axons within Gr medially near the level of obex (Ding et al. 1999, Ueyama et al. 1985, 1987). More Gr labeling was found in response to the application of HRP to the cut end of the perineal nerve branch than to the DNP sensory branch (Ueyama et al. 1985, 1987). An extensive search of the Gr using extracellular recordings in urethane-anesthetized animals (Cothron et al. 2008) revealed only a small proportion of electrode tracks (41 of 319 in 12 rats) in the medial third of the nucleus around obex containing neurons responsive to penile stimulation. These neurons did, however, also respond to stimulation of a variety of somatic territories, but not the distal colon (Fig. 8.2). The lack of responses to colon is probably related to the medial location of the penile-responsive neurons in Gr. In another electrophysiologic recording study of somatovisceral interactions in the Gr of male rats (Rong et al. 2004), none of the 43 neurons (out of 212 tested) that were excited or inhibited by colorectal distension responded to stimulation of the scrotum (i.e., the most common somatic convergent territory for penile-responsive neurons). The somatic receptive fields for the Gr neurons responding to colorectal distension were centered on the outer leg from the hip to the foot, and tended to be located lateral and caudal relative to obex. Of particular note is the finding, using both electrophysiological and neuroanatomical tracing techniques, that penile inputs to Gr are both ipsi- and contralateral, whereas cutaneous inputs are only ipsilateral (Cothron et al. 2008).
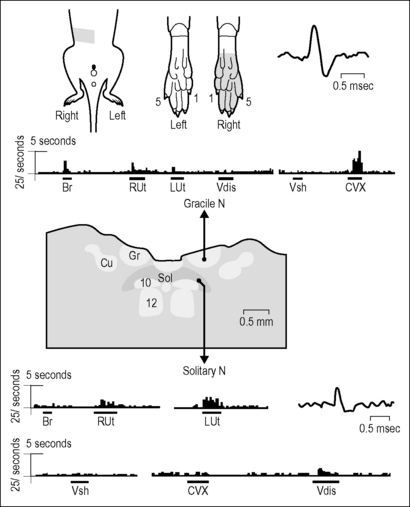
Interestingly, although a study in cats also found penile projections to Gr, the inputs from the clitoris in females were not in an analogous position (Kawatani et al. 1994). Many neurons in the rodent Gr have been shown to receive input from the female internal reproductive organs and colon (Berkley & Hubscher 1995a, Bradshaw & Berkley 2000, Hubscher 1994). In addition, the responses to reproductive organ stimulation (cervix pressure, vaginal and uterine distension) vary across the estrous cycle (latencies and proportion of excitatory/inhibitory responses), suggesting a possible role in mating (Bradshaw & Berkley 2000). The hormonal influences are probably due to fluctuations in levels of 17β-estradiol (Bradshaw & Berkley 2003). The most common somatic receptive fields for Gr neurons responding to stimulation of the female internal reproductive organs are the ipsilateral foot/toes, followed by the leg (including ankle), tail, and perineum. A typical example showing a somatovisceral convergent neuronal response in Gr is provided in Figure 8.3. Note that the foot/toes were also a common convergent territory for female reproductive organ responsive neurons at the thoracolumbar and lumbosacral levels of the spinal cord, albeit bilateral.
The solitary nucleus
The solitary nucleus (NTS) is another region within the caudal medulla (just below Gr) that receives primary afferent inputs. However, unlike Gr, which receives primary inputs from small cutaneous receptive fields, the NTS receives visceral and pelvic organ inputs. The NTS is known as a region that processes gustatory, cardiovascular, and respiratory information as well as information from the esophagus, stomach, cecum, small intestine, colon, cervix, uterus, and vaginal canal, with a general viscerotopic organization from rostral (gustatory) to caudal (pelvic organs) (Altschuler et al. 1989, 1991, 1993, Cechetto 1987, Collins et al. 1999, Hubscher & Berkley 1994, Jean 1991, Lu & Bieger 1998, Norgren & Leonard 1973, Novin et al. 1981, Zhang et al. 1992).
The NTS receives primary afferent input via the nodose ganglion from viscera innervated by the vagus, including those within the pelvic region (via the abdominal branches of the vagus (Altschuler et al. 1993, Gabella & Pease 1973, Ortega-Villalobos et al. 1990)). For example, a vagal–solitary projection from the uterus has been demonstrated with neuroanatomic tracing and other types of animal experiments (Collins et al. 1999, Guevara-Guzman et al. 2001, Ortega-Villalobos et al. 1990). Electrophysiological studies in rats indicate NTS neurons responsive to stimulation of the pelvic viscera, including the cervix, vaginal canal, uterus, and colon (Hubscher & Berkley 1994). Of the responsive neurons, 29% respond to more than one visceral organ, demonstrating the existence of viscerovisceral convergence and hence the potential for interactions between organ systems. Consistent with the literature was the finding that none of the NTS neurons responded to stimulation of somatic territories (no somatovisceral convergence). A typical example illustrating this point (and the contrast with the nearby Gr) is presented in Figure 8.3.
The NTS has also been shown to be a relay of inputs coming from the spinal cord. Retrograde tracing from the NTS indicates that neurons comprising the spinosolitary pathway include cell bodies located in the superficial dorsal horn, laminae IV–VII, the dorsal commissural nucleus of lamina X (thoracolumbar levels), and within the vicinity of the sacral parasympathetic nuclear region (Esteves et al. 1993, Menetrey & Basbaum 1987, Menetrey & de Pommery 1991). Lesion studies indicate that different pathways probably exist for different portions of the reproductive tract. Electrophysiological recording of responses to pelvic organ stimulation in the NTS pre and post combinations of acute complete spinal transection and bilateral vagotomy were made (Hubscher & Berkley 1995). The lack of responses to uterine distension post vagotomy is consistent with anatomic tracing and other studies (Collins et al. 1999, Guevara-Guzman et al. 2001, Ortega-Villalobos et al. 1990). In addition, although bilateral vagotomy had an effect on the response properties of neurons in the NTS for cervix/vaginal stimulation, suggestive of an anatomical connection (Hubscher & Berkley 1995, Komisaruk & Sansone 2003), the responses were only eliminated after a subsequent spinal transection (Hubscher 1994, Hubscher & Berkley 1995).
Major central relays of viscerosomatic convergent inputs
Medullary reticular formation
The medullary reticular formation (MRF) is a region in the rostral medulla of the brain stem that is known to contribute to numerous body functions. These functions include ascending control of cortical arousal, descending control of motor activity, and the control of autonomic activity (Jones 1995). The MRF in the rat has been shown to have direct reciprocal interconnections with all three ports of entry described in the previous section (spinal cord, Gr, NTS) (Aicher et al. 1995, Antal et al. 1996, Basbaum & Fields 1979, Casey 1969, Chaouch et al. 1983, Gallager & Pert 1978, Hermann et al. 2003, Jean 1991, Mtui et al. 1995, Odutola 1977, Tomasulo & Emmers 1972). Thus, MRF neurons receive convergent inputs from multiple somatic and visceral territories, which include regions innervated by spinal as well as cranial nerves. The MRF is known to process and relay a vast array of viscerosomatic sensory inputs involved in nociception (Bowsher 1976, Chan 1985, Hubscher & Johnson 1996, Peschanski & Besson 1984, Zhuo & Gebhart 1990). Many MRF neurons in both male and female rats, for example, respond to distension of the distal colon (Hubscher 2006b, Kaddumi & Hubscher 2006). All of the colon-responsive single MRF neurons receive bilateral nociceptive-specific somatic inputs from widespread regions of the body.
The MRF has been implicated to be involved in the neural circuitries mediating eliminative functions, including urination, defecation, and ejaculation. For urogenital processing in male rats, electrical stimulation of the MRF has been shown to produce field potentials in the lumbosacral spinal cord near pudendal motor nuclei (Tanaka & Arnold 1993), reduce pudendal motor neuron reflex discharges (Johnson & Hubscher 1998), and activate postganglionic sympathetic fibers contained within the motor branch of the pudendal nerve (Johnson & Hubscher 2000). Large MRF lesions that include the ventral nucleus reticularis gigantocellularis, gigantocellularis pars alpha, and lateral paragigantocellular nuclei affect ejaculatory bursts in perineal muscles (Marson & McKenna 1990).
Electrophysiological data from extracellular single unit recordings in the rostral ventromedial medulla in male rats has demonstrated a significant degree of viscerosomatic and viscerovisceral convergence. Somatic convergent territories are not just confined to the hind quarters but include rostral skin areas such as the ears and forepaws (Hubscher 2006b, Hubscher & Johnson 1996, Kaddumi & Hubscher 2006). Many of these viscerosomatic MRF neurons receive input from cutaneous territories across the entire body, including the face. The majority of the viscerosomatic neuronal responses are to noxious levels of stimulation. There is also a high degree of viscerovisceral convergence in the MRF. Single unit recordings in MRF indicate that 62% of neurons responding to distension of the urinary bladder also respond to stimulation of the urethra, penis, and distal colon (Hubscher 2006b, Kaddumi & Hubscher 2006), suggesting that this CNS region is probably important for the coordination of visceral functions.
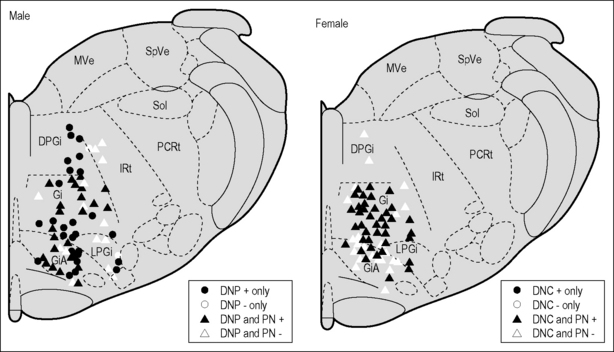
Viscerosomatic convergent neurons in the MRF in female rats respond to electrical stimulation of the dorsal nerve of the clitoris, which is the sensory pudendal branch equivalent of the DNP in males. A summary illustrating the convergence of pudendal/pelvic nerve inputs at one anteroposterior level of the MRF is presented in Figure 8.4. Note the predominance of the pelvic nerve input in females, which probably reflects the importance of the vagino- cervical responses to mating. This region has been shown to be involved in female circuitry responsible for the lordosis mating posture (Daniels et al. 1999, Modianos & Pfaff 1979, Schwartz-Giblin et al. 1996). As with the reproductive organ inputs to Gr, there are estradiol-associated differences in the responses of MRF neurons to viscero-somatic stimulation (Hubscher 2006b). Most interesting was the finding that the hormonal response variations for somatic and visceral territories were in opposite directions (estradiol-associated hypersensitivity and hyposensitivity, respectively), which is consistent with what is expected during mating (for example, the reduced sensitivity of the cervix). The absence of hormonal effects for some of the viscerosomatic inputs combined with different effects depending on the source for the same individual neurons suggests that estradiol is acting elsewhere, such as within the spinal cord itself (see Hubscher 2006b).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree