Chapter 2 Cartilage Repair and Regeneration
Cartilage structure and function
The Chondrocyte as an Anabolic/Catabolic Cell
Chondrocytes are highly specialized cells that differentiate from clusters of mesenchymal cells during skeletal embryogenesis. The chondrocyte synthesizes and secretes the components of the extracellular matrix, primarily proteoglycans and type II collagen. Most of the immature cartilage is temporary and is replaced by bone during epiphyseal development, whereas the regions nearest the synovial cavity remain as the permanent articular cartilage of the adult. During growth and development, immature cartilage undergoes cellular replication in both the superficial and deep zones. However, as skeletal maturity approaches, replication occurs only in the deep zone. Cell replication after skeletal maturity is rare. The cell content of articular cartilage is low, occupying no more than 10% of the tissue volume in humans. Cell density has been estimated as 105 cells per cubic millimeter in newborns and 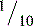 of that in adult cartilage. Values are higher in the superficial than in the deeper zone. Experimental animals have far greater cellularity. For example, adult rabbits have nearly 10-fold greater cell density than human cartilage, and mice have 25-fold greater cell density.1 The general cellular morphology ranges from flattened and discoidal in the most superficial zones to ovoid in the deeper regions. The ovoid cells display enlarged Golgi bodies, a characteristic of cells actively secreting proteins, and cellular processes that extend into the adjacent pericellular matrix.
of that in adult cartilage. Values are higher in the superficial than in the deeper zone. Experimental animals have far greater cellularity. For example, adult rabbits have nearly 10-fold greater cell density than human cartilage, and mice have 25-fold greater cell density.1 The general cellular morphology ranges from flattened and discoidal in the most superficial zones to ovoid in the deeper regions. The ovoid cells display enlarged Golgi bodies, a characteristic of cells actively secreting proteins, and cellular processes that extend into the adjacent pericellular matrix.
Chondrocytes are dynamic cells with anabolic and catabolic activity; they mediate both synthesis and degradation of the matrix. Proteoglycan metabolism has been studied extensively. As is typical for other cells, the protein components are synthesized in the cytoplasmic rough endoplasmic reticulum, and sulfation of the polysaccharides occurs in the Golgi bodies. Ex vivo studies with radioactive tracer isotope of 35S-sulfate show incorporation into glycosaminoglycans by intermediate and deep cells and subsequent movement into the matrix. Type II collagen is synthesized and secreted as separate procollagen chains with extensions on its ends, converted to tropocollagen, and organized into fibrils with small amounts of type IX and type XI collagen. In contrast to the dense, thick, highly oriented collagen fibers of bone, cartilage fibrils are thin and cross-linked into an open meshwork. The fibrils contain variable amounts of noncollagenous macromolecules, notably decorin. The half-life of type II collagen is more than 200 years in humans. Thus, the major component of the native fibrils does not appear to be renewed or reparable in the normal setting. The collagen fibrillar network is under tension and serves to contain the glycosaminoglycans in a compressed state. It is difficult to imagine how that network could be turned over without compromising the mechanical integrity of the tissue or how denatured foci could be mended. Evidence shows damage to the fibrillar network in osteoarthritis. Osteoarthritis drastically affects the mechanical properties of cartilage. Excessive swelling of osteoarthritic samples in dilute salt solution is taken as evidence of the loss of resistance afforded by the fibrillar net to absorption of water by the polysaccharides. Although there appears to be little turnover of the fibrillar network, the entrapped proteoglycans undergo turnover that can be accelerated by local cytokines. Chondrocytes are responsible for maintaining the matrix environment in which they are encased and hence ensure the tissue’s mechanical characterics.2 They are protected from osmotic and mechanical damage by the rigid pericellular matrix, called the chondron. Maintenance of the matrix involves degradation by proteinases and free radicals generated by the chondrocyte. Matrix metalloproteinases and aggrecanase catalyze the turnover of cartilage matrix in normal as well as in diseased cartilage. Because many of the matrix components in cartilage are specific to that tissue, there is great interest in developing and validating assays for their degradation products in plasma or synovial fluid as markers for turnover.3
Mechanical properties of articular cartilage
Articular cartilage is a hypocellular, viscoelastic tissue that lines synovial joints, providing them with a nearby frictionless environment. Synovial cartilage articulations provide a coefficient of friction for joint motion that is less than 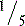 that of ice on ice.4 The mechanical properties of articular cartilage depend upon its composition and its architecture. Normally, the hydrophilic proteoglycans and collagen constitute 30% of the tissue mass; the remainder is water. Cartilage matrix can be viewed as a biphasic material in which the fluid phase flows upon mechanical deformation of its solid phase. Although the water is constrained by the proteoglycan molecules, the fluid phase can also be called the porosity of the cartilage. The high water content of the tissue also generates its high viscoelasticity. Its elastic modules is low at slow rates of loading but is two orders greater at physiologic rates.
that of ice on ice.4 The mechanical properties of articular cartilage depend upon its composition and its architecture. Normally, the hydrophilic proteoglycans and collagen constitute 30% of the tissue mass; the remainder is water. Cartilage matrix can be viewed as a biphasic material in which the fluid phase flows upon mechanical deformation of its solid phase. Although the water is constrained by the proteoglycan molecules, the fluid phase can also be called the porosity of the cartilage. The high water content of the tissue also generates its high viscoelasticity. Its elastic modules is low at slow rates of loading but is two orders greater at physiologic rates.
The surface cartilage layer or “skin” is resistant to compressive loads or penetration. The vertically arranged collagen fibers of the radial and calcified zones are resistant to shear. Upon application of pressure to articular cartilage through weight bearing, the water contained within the cartilage exudes upon pressure. With diminished pressure, water is drawn back to the aggrecan. The surface protein dermatan sulfate also acts as an antiadhesion substance. The fine filaments of the superficial zone combine with water so that articulation with the opposite joint surface also occurs with combined water and superficial zone filaments.2 Therefore, the lubricating barrier between joint surfaces is mostly water. Water is released during weight-bearing pressure from hyperhydrated negatively charged proteoglycans in articular cartilage. With damage or degeneration, loss of proteoglycans and water results in impaired mechanical properties and joint function.
Incidence of Cartilage Lesions
The true incidence of cartilage lesions and their natural history are unknown. It has been proposed that between 5% and 10% of acute knee hemarthroses after a work-related or sports injury is associated with an acute chondral injury.5 In a retrospective review of 31,516 knee arthroscopies, the prevalence of chondral lesions was 63%. However, isolated unipolar chondral defects in patients younger than 40 years were rare, occurring in only 5% of this patient population.6 Both clinical and experimental evidence showed that with time focal cartilage injuries will enlarge and progress to osteoarthritis.7
Mechanical injury to articular cartilage during sporting injuries may occur with shearing forces secondary to disruption of the anterior cruciate ligament. Shearing osteochondral fractures occurring at the time of ligament disruption have been noted. Blunt injury to the joint surfaces may cause injury to and death of articular chondrocytes. If the articular chondrocyte cannot continue to synthesize and remodel its matrix macromolecules, the pericellular matrix eventually will degenerate. This may account for the high incidence of osteoarthritis encountered with anterior cruciate ligament injuries. Acutely the incidence of chondral injuries is approximately 2% but may approach 20% in the long run.8
In a study performed by Repo and Finlay,9 blunt force to articular chondrocytes in excess of 25 MPa reproducibly resulted in death of articular chondrocytes. Hence there appears to be a threshold to which articular chondrocytes can withstand blunt trauma. This may be an important factor in understanding articular cartilage degeneration after injury and may be an important technical factor during new repair techniques, such as osteochondral graft transfers. Large impaction forces needed to introduce osteochondral grafts to recipient sites may result in injury and cell death to the cartilage cap of the osteochondral grafts, leading to failed long-term results.
Magnetic resonance imaging scans demonstrated bone bruises after blunt injuries sustained during work-related and sporting activities. Arthroscopic biopsy studies of cartilage overlying bone bruises demonstrated superficial chondrocyte death and matrix dehydration.10 Cartilage cell death is proposed to arise directly from the blunt trauma exceeding this threshold.
The natural history of osteoarthritis itself is unknown. A Swedish longitudinal study notes radiographic progression of osteoarthritis in the knee occurs over a 20-year time course when greater than 50% joint space narrowing is present at initial evaluation (Ahlback stages 2–4).11 However, only 60% of patients with Ahlback stage 0 (peripheral osteophytes and a normal joint space) or Ahlback stage 1 (<50% joint space narrowing) at initial presentation will progress radiographically. Not all radiographic osteoarthritis will progress.
Cartilage Injuries and Repair
Clinical and experimental evidence shows that damage involving the articular cartilage surface and confined to the cartilage undergoes little restoration. Cartilage has little intrinsic ability to heal. Chondrocytes in mature articular cartilage rarely divide, and their density declines with age. In contrast, lesions that extend to the subchondral marrow may heal clinically.12 Therefore, a cell source for cartilage regeneration or repair must arise from the underlying subchondral bone marrow, the adjacent synovial tissue, or an exogenous source.
Cartilage repair is dependent on the mobilization of cells derived from the subchondral bone marrow, which include multipotential cells, osteoblasts, chondroblasts, fibroblasts, and hematoprogenitor cells.13 Therefore, the repair tissue that results may be variable dependent based on the predominant cell line that proliferates and its modulation by local growth factors, cytokines, and the local mechanical environment.
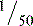 that of kidney, but the rates of glycolysis between chondrocytes and kidney are comparable. Thus, chondrocytes engage in relatively anaerobic metabolism.
that of kidney, but the rates of glycolysis between chondrocytes and kidney are comparable. Thus, chondrocytes engage in relatively anaerobic metabolism.








