20
Cartilage Injuries in the Shoulder
Localized articular cartilage lesions of the glenohumeral joint in young athletes are a rare occurrence and are generally well tolerated. When symptomatic, however, they can be quite painful and limiting. Treatment recommendations remain difficult because there is little information to glean from our experience or from the literature to guide the decision-making process. Clearly, some analogies can be extrapolated from a relatively mature treatment algorithm used to treat chondral injury of the knee.1–4 At this juncture, however, the application of existing and emerging technologies is largely anecdotal or relegated to case reports. This chapter provides the orthopaedic surgeon with guidance in treating these problems by summarizing the information available on this topic, and discusses how contemporary treatment in other joints may be applicable to the glenohumeral joint. This chapter does not address degenerative arthritis as seen in older individuals.
 Classification and Etiology
Classification and Etiology
There is no classification scheme designed specifically to describe articular cartilage lesions in the shoulder. The Outerbridge system5 is one classification system commonly used in the knee and is acceptable for use in the shoulder as a tool to describe the general appearance of a chondral lesion. Grade 0 is normal cartilage, grade I is articular cartilage softening, grade II is fibrillation involving half the depth of the articular surface, grade III is fissuring involving more than half the depth of the articular surface, and grade IV is full-thickness loss reaching to or through the subchondral bone. Other factors such as location (glenoid or humerus), position (central or peripheral), size, degree of containment, depth (chondral or osteochondral), etiology (avascular necrosis, localized degenerative or posttraumatic), and defects that develop following surgical intervention (i.e., shoulder stabilization) also factor into the decision-making process and provide a common language to communicate the nature of these lesions.
 Incidence
Incidence
The incidence of articular cartilage lesions in the shoulder is unknown. Due to issues related to impact loading and weight bearing, one would expect it to occur less frequently than in the knee joint. It is probable that articular cartilage lesions of the glenohumeral joint are more likely to remain asymptomatic and quiescent relative to their counterparts that occur in the knee and therefore are more likely to remain unrecognized clinically. The authors believe that the incidence of these lesions in the knee is at best considered a worst-case scenario of what might be occurring in the shoulder.
Curl et al6 reviewed 31,516 knee arthroscopies. They reported on the incidence of grade III lesions (41%) and grade IV lesions (19%). In patients less than 40 years of age, the incidence of grade IV lesions was only 5%. Hjelle et al7 performed a prospective study consisting of 1000 patients and similarly found a 5% incidence of grades III and IV chondral defects. It must be understood that only a small percentage of these lesions were clinically symptomatic requiring treatment.
One small study using magnetic resonance arthrography (MRA) found glenohumeral cartilage lesions in up to one third of patients.8 Some studies describe the incidence of chondral injury in association with specific pathology. In a cadaveric study, Hsu et al9 demonstrated that the area of articular cartilage damage to the glenoid and humeral head in specimens with associated rotator cuff tears was 32% and 36%, respectively. This compared with 6% in the glenoid and 7% in the humeral head in specimens without rotator cuff tears. The location of the degeneration tended to be in the anterioinferior glenoid and posterior humeral head, and the degree of damage in the glenoid was correlated with that of the humeral head. Gartsman and Taverna10 identified only nine significant cartilage lesions (grade IV) in 200 arthroscopies (4.5%) in association with full-thickness rotator cuff tears as incidental intraarticular abnormalities at the time of rotator cuff repair.
The incidence of chondral injury in patients with shoulder instability is well documented. Recently, Werner et al11 demonstrated that patients with traumatic and atraumatic shoulder instability who came to surgery had approximately an 80% incidence of Hill-Sachs lesions in addition to chondral damage of the glenoid in many instances. Typically, in patients with atraumatic shoulder instability who respond to therapy, the incidence of Hill-Sachs lesions is significantly lower compared with patients with traumatically induced shoulder instability. In first-time traumatic dislocators, Taylor and Arciero12 reported that 90% of the patients had a Hill-Sachs lesion, with 40% classified as a chondral lesion and 60% classified as an osteochondral lesion. In a large retrospective review published by Cameron et al,13 the extent and presence of grade III and IV damage was associated with the time from injury to surgery. Patients with glenohumeral chondral damage were also significantly older than those without chondral damage (34.9 years versus 29.6 years). Hintermann and Gachter14 reported a 23% incidence of glenoid and 8% incidence of humeral head degenerative arthritis in patients who sustained only one dislocation. This compared with a 36% incidence of humeral head and 27% incidence of glenoid degenerative arthritis in patients who sustained two or more dislocations.
The natural history of these lesions with or without associated pathology is completely unknown. The senior author (B.J.C.) believes that most chondral and osteochondral injuries of the glenohumeral joint are well tolerated. However, once they become symptomatic, they are unlikely to revert back to a quiescent state without effective treatment.
 Patient Evaluation
Patient Evaluation
A thorough history and physical examination is the first step in the decision-making process. The mechanism of injury and the chronicity of the symptoms are the initial factors determining the etiology of the patient’s symptoms. A history of direct shoulder trauma, traumatic or atraumatic glenohumeral instability, or previous intraarticular surgery is a relevant issue to -explore. Patients may complain of mechanical symptoms, aching that may vary with barometric pressure change, discomfort at night, difficulty sleeping on the affected side, and pain with glenohumeral motion during activities of daily living. It is important that the surgeon obtain a realistic appraisal of the quality and severity of the patient’s symptoms and discuss with the patient to what extent these symptoms interfere with daily activities as well as with higher level demands such as recreational or competitive sports. More than any other variable, managing patient expectations is critical to the ultimate success of the intervention chosen.
Physical examination includes assessment for atrophy, active and passive motion, scapulothoracic dyskinesis, stability testing, special testing to assess for labral pathology, and compression-rotation testing to assess for mechanical symptoms and pain. Unlike patients with osteoarthritis, these patients rarely have significant limitations in passive and active motion. The presence of motion loss is consistent with soft tissue contractures. Obvious cofactors, such as instability, superior labral anterior to posterior (SLAP) tears, and rotator cuff pathology, are usually apparent due to the patient’s history and classic findings on examination.
Plain radiographs should be obtained consisting of a true anteroposterior, scapular-Y view, and an axillary view. Additional views such as the West Point axillary view, Stryker notch view, and supraspinatus outlet view facilitate more precise anatomic delineation. We commonly request magnetic resonance imaging (MRI) to evaluate the articular surface and to evaluate for any other intraarticular abnormalities. Carroll et al15 have reported on MRI’s use in detecting articular cartilage lesions on the humerus. The sensitivity varied between radiologists ranging from 53 to 100% and the specificity ranged from 51 to 87%.8 Unfortunately, lesions can be missed by MRI as was demonstrated by Cameron et al,16 where 45% of grade IV chondral lesions had no radiographic (MRI or plain radiographs) evidence preoperatively.
The same techniques used to identify chondral injuries in the knee are useful for the glenohumeral joint. Standard pulse sequences for articular cartilage frequently include a T2-weighted image with or without fat suppression and a T1-weighted fat-suppressed three-dimensional spoiled gradient-echo technique. These protocols benefit from the arthrogram-producing effect of the differential signal intensity on the joint fluid, which highlights irregularity or defects of the joint surface. On the T1-weighted images, cartilage is higher in signal intensity than joint fluid; the reverse is true for the T2-weighted images (Fig. 20–1).17–20 A computed tomography (CT) scan is useful in situations where a significant osteochondral defect exists such as that following posttraumatic instability (Fig. 20–2).
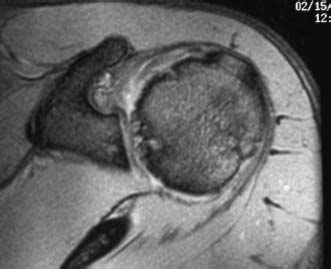
FIGURE 20–1 Axial-oblique T2-weighted magnetic resonance imaging (MRI) demonstrating significant articular cartilage loss involving primarily the humeral head with some subchondral cystic changes evident.
It is not uncommon for patients in this age group (i.e., less than 40) to have concomitant pathology in addition to their chondral injury. Most commonly, these lesions are associated with a history of traumatic shoulder instability, SLAP tears, and rotator cuff pathology. Addressing these cofactors first with traditional treatment modalities is prudent, as it is likely that the associated chondral injury is nothing more than an incidental finding that will remain asymptomatic. However, the patient’s symptom complex and history may provide an indication preoperatively to consider at least some form of initial treatment of these lesions (i.e., debridement,marrow stimulation) at the time when the patient’s primary pathology is addressed.
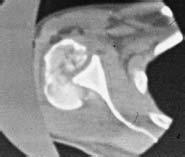
FIGURE 20–2 Computed tomography (CT) scan of a patient with a posterior fracture-dislocation involving the majority of the humeral head.
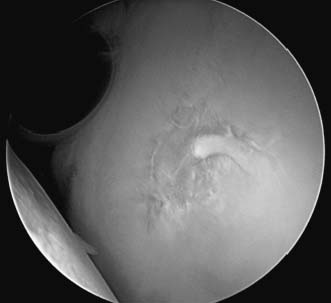
FIGURE 20–3 Arthroscopic view of a normal glenoid demonstrating the central-most portion to be nearly devoid of articular cartilage.
Arthroscopic evaluation of chondral injury is the most useful determinate as these lesions are generally occult when documented at the time of index treatment. The lesion itself must be carefully defined. Generally, the central portion of the glenoid is normally quite thin and may actually be translucent to the extent that subchondral bone is exposed (Fig. 20–3). It is also important to avoid confusing the bare area of the humeral head (Fig. 20–4) with a chondral lesion. This is an area of bare bone with remnants of old vascular channels. This area is juxtaposed with the rotator cuff insertion and completely devoid of cartilage on its most lateral extent. This bare area also correlates with the attachment of the infraspinatus tendon and can be used as a landmark in rotator cuff surgery to align the infraspinatus to its footprint.
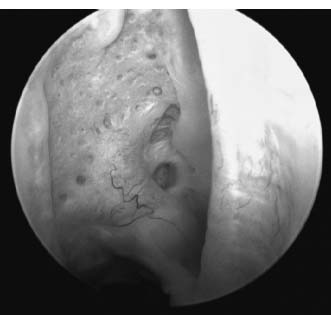
FIGURE 20–4 Arthroscopic view of the normal bare area of the humeral head.
Finally, the topography of the articular cartilage thickness of the humeral head has been studied by the authors in our laboratory and demonstrates mean thickness that ranges from 0.6 to 1.2 mm. The cartilage is thicker centrally and thins toward the periphery.21 Although important, at this juncture, this finding has indeterminate significance when deciding on which treatment option is ultimately implemented.
Visualizing the lesion from opposite portals and during arm positioning facilitates a comprehensive evaluation of the defect, which is otherwise difficult to perform from a single portal due to the spherical nature of the humeral head. Palpating the lesion with a probe is important as the extent of the defect typically is beyond visible areas of exposed bone and should be documented to include any degenerated margins at the transition zone adjacent to healthy appearing cartilage. Measuring the lesion with a probe tip of specific length or a graduated instrument provides sizing to determine the surface area and depth of the defect.
 Nonoperative Treatment
Nonoperative Treatment
As with any shoulder pathology, conservative treatment is initially justified and should include nonsteroidal antiinflammatory medications, physical therapy, glenohumeral steroid injections, and consideration for injectable viscosupplementation with hyaluronic acid compounds. Physical therapy should emphasize scapulothoracic and glenohumeral strengthening. Often, these patients have non-outlet or secondary impingement due to abnormal scapulothoracic kinematics. This can be predictably improved on with appropriate rehabilitation. Steroid injections may be appropriate, but we do not favor repeated use in younger patients. Patients typically become symptomatic once they resume physical activities. Finally, viscosupplementation, although currently approved for use in the knee, is considered for off-label usage in the shoulder and must be performed with explicit patient informed consent. The use of viscosupplementation is a promising alternative for patients with overt glenohumeral arthritis and is the subject of prospective study. However, similar to steroid injections, we rarely use this in the younger patient population with localized chondral disease.
 Operative Treatment
Operative Treatment
Virtually any cartilage restoration option used in the knee can be applied to the glenohumeral joint.22 There is a treatment continuum to consider when deciding which procedure to perform. The nature of these treatment options can be subcategorized into primary treatment, palliative treatment, reparative treatment, and restorative treatment. Primary treatment consists of repair of the cartilage. Palliative treatment consists of arthroscopic lavage and debridement. Reparative treatment consists of marrow stimulation techniques (abrasion, drilling or microfracture). Restorative options include osteochondral grafting (autograft and fresh allograft) and autologous chondrocyte implantation.
Decision Making
The decision making must take into account several factors. Treating obvious comorbidities first is essential, as most defects do not manifest in symptoms following repair of associated pathology such as rotator cuff tears, SLAP tears, or the pathoanatomy associated with instability. Initially, the presence of symptoms, not the mere identification or knowledge of an existing defect, must guide the decision to implement one of the treatment alternatives. Knowledge of previous treatments provided, the rehabilitation performed postoperatively, and the patient’s response to this treatment facilitates decision making, as repeating the same option again is intuitively less desirable. In addition, managing and matching patient expectations must be considered in the context of the magnitude of improvement that might be expected with each treatment option.
Because the data available and our experience with these lesions is relatively limited, we recommend that initial treatment options be limited to those that will not compromise the implementation of future treatment options should they become necessary. In all situations, associated stiffness is managed with arthroscopic release with gentle manipulation. An examination under anesthesia enables the surgeon to ascertain the area of tightness and determine which portion of the capsule should be arthroscopically released. Subsequent decision making is implemented in the context of several patient- and defect-specific factors.
Synthesizing the findings from the patient’s history, symptoms, and findings on examination may help to ascertain the exact etiology of the patient’s pain and the subsequent level of treatment required. For example, pain at the extremes of motion may be due to capsular contracture and synovitis. Similarly, pain at rest may indicate synovitis. These patients may respond more favorably to guided capsular release and synovectomy. Pain from articular cartilage abnormalities more commonly occurs in the midranges of motion and may be associated with mechanical symptoms and crepitus made worse with loading of the glenohumeral joint. These patients may require specific attention to the articular cartilage defect to improve their function and reduce their pain.
The magnitude of the patient’s symptoms is also a consideration, as first-line treatment with debridement and lavage or microfracture may suffice in patients with low-level symptoms or those who place relatively low physical demands on their shoulder. Similarly, the morbidity of the operation may be a factor considered by the patient in the decision making. Unlike autologous chondrocyte implantation, which requires a biopsy from the knee (preferred by the senior author, B.J.C.), or osteochondral autografting, which also requires harvesting of an osteochondral plug from the knee, fresh osteochondral allograft transplantation is a single-stage procedure limited to the glenohumeral joint, which may be preferable to some. Patients with large, uncontained defects with subchondral bone loss, independent of age, are excellent candidates for fresh osteochondral allograft transplantation. Patients with smaller contained superficial defects, especially when relatively young, are excellent candidates for autologous chondrocyte implantation.
Incidentally discovered defects that might be considered as a relative contributor to the patient’s symptoms may be addressed at the same time any associated pathology is corrected (i.e., rotator cuff tears, SLAP tears, and instability patterns) with a marrow stimulation technique. Patients with deeper defects may more commonly complain of mechanical symptoms, and therefore will likely respond more favorably to solutions that can restore articular congruity such as osteochondral grafting. Smaller defects (i.e., less than 10 mm2) may be amenable to osteochondral autograft transplantation, and larger defects may be more amenable to fresh osteochondral allograft transplantation.
Superficial lesions or surface lesions with minimal subchondral bone involvement, which fail first-line treatment using debridement and lavage or marrow stimulation, especially in young patients, are considered for revision with autologous chondrocyte implantation. The most difficult decision is for older patients with superficial defects. Because of the morbidity of two surgical procedures and the prolonged rehabilitation required of autologous chondrocyte implantation, we favor fresh osteochondral allograft in this population because if it fails, revision with arthroplasty is a plausible alternative.
The most advanced scenario includes young and active patients with opposing defects of the glenoid and humerus (i.e., bipolar disease). Clearly, this is the most challenging patient group and often requires salvage solutions as alternatives to arthroplasty. In this population, regaining motion is the first objective, and typically patients are managed initially with arthroscopic capsular release, lavage and debridement, and possibly microfracture of exposed surfaces. Second-line treatment options include biologic resurfacing with the posteriorly reflected anterior shoulder capsule, soft tissue allografts, porcine small intestine submucosa (SIS), and meniscal allografts. These solutions may improve the patient’s function and reduce pain without compromising future arthroplasty reconstruction.
 Primary Treatment
Primary Treatment
Osteochondral fractures of the shoulder can occur following high-energy trauma and are most frequently seen with fracture dislocations of the shoulder (Fig. 20–2). In relatively young patients, open reduction, fragment elevation, bone grafting, and fixation are attempted if possible. We recommend intraarticular fracture fixation with headless compression screws (Acumed, Inc., Beaverton, OR) that do not require removal.
Osteochondritis dissecans of the humeral head is a rare condition with only a few case series reported in the literature.23–29 It is not uncommonly seen in relatively active individuals as the etiology is related to trauma with associated ischemia, although endocrine abnormalities, genetic influences, and the presence of anomalous centers of ossification have also been attributed to this entity.23,24 Most commonly, it involves the humerus, but rarely may involve the glenoid.25 It more commonly affects middle-aged and young males and typically involves the anterosuperior aspect of the humeral head.26 Management may include drilling,27,28 drilling with fragment removal,26 or primary repair. Because of the risk for progressive degeneration following fragment removal, we recommend treatment with debridement, microfracture of the base, and primary repair using screws or bioabsorbable pins (Fig. 20–5).
 Palliative Treatment
Palliative Treatment
Lavage and debridement improve congruence and remove debris and various enzymes and proteins such as metalloproteinases associated with the pathogenesis of arthrosis. The indications for arthroscopic lavage and debridement is as a first-line treatment and the outcome is technique and disease-level dependent. The principal components of arthroscopic debridement may include synovectomy, removal of loose bodies, and judicious use of capsular release for motion loss. In addition, if preoperative and arthroscopic findings implicate the subacromial space as a source of pain, a formal subacromial decompression should also be performed as supported by earlier studies.30,31
The objectives for the management of chondral and osteochondral defects include converting edges with gradual transition zones to those with vertical margins. The basic science supporting this technique was studied by Rudd et al,29 with the recommendations from this investigation being widely accepted as a modality to manage cartilage defects of any joint. In a canine model, humeral defects prepared with and without beveling of the margins of articular cartilage defects were compared over a 16-week period. The authors concluded that cartilage defects with beveled edges were significantly larger than those with perpendicular edges. In addition, chondral damage to the glenoid surface occurred more frequently opposite beveled defects than opposing defects with vertical walls. Thus, if one chooses to modify the defect in any fashion, the combined use of curettes, motorized shavers run in a single direction, and arthroscopic hand instruments can change a gradual transition zone from the defect to the surrounding normal cartilage to one with a vertical wall of healthy articular cartilage. Intuitively, defects prepared in this fashion should be better suited to share load and provide relative protection to exposed subchondral surfaces.
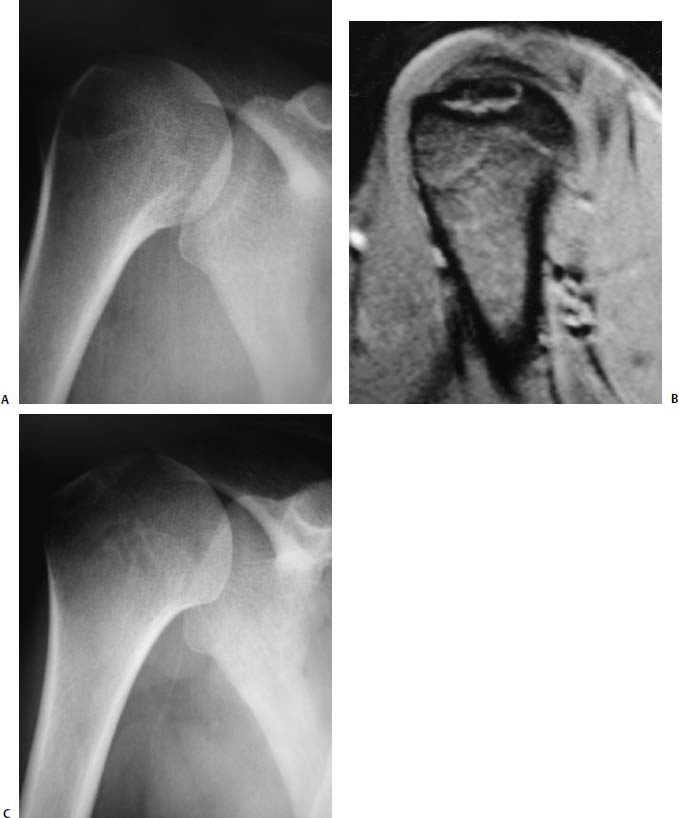
FIGURE 20–5 Radiographic series of a patient with osteochondritis dissecans of the humeral head demonstrated on preoperative radiograph (A) and MRI (B) treated successfully with drilling and bioabsorbable pin placement with demonstrable healing on postoperative anteroposterior radiograph (C) obtained at 12 months.
Thermal chondroplasty should be used with great caution. It can result in a wide area of chondrocyte cell death, which might affect the subchondral bone, especially given the relatively thin articular surfaces of the glenohumeral joint.32
Similar to the results following arthroscopic lavage and debridement in the knee, the literature in the shoulder largely consists of patients with glenohumeral osteoarthritis, and the results tend to be relatively short-lived and incomplete.16,33–39 The most relevant study to date is that published recently by Cameron et al.16 In this investigation of patients diagnosed with shoulder instability, 61 patients with humeral or glenoid grade IV lesions were treated with arthroscopic debridement and capsular release where necessary. Eighty-seven percent of the patients indicated that they would have the surgery again. Pain relief was obtained in 88% with a mean duration of pain relief of 28 months. Patients also demonstrated reductions in rest pain, and pain during light and strenuous activities. Negative prognostic factors leading to the return of pain and failure of the procedure included lesion size of greater than 2 cm2. This is analogous to the cutoff used in the knee when lesion size is factored into the treatment algorithm.1,3
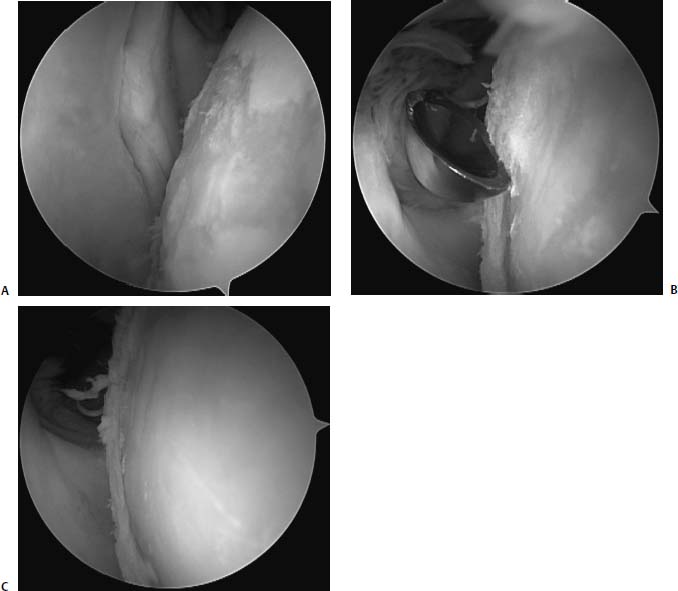
FIGURE 20–6 Arthroscopic photographs of a 28-year-old man with a focal chondral defect of the humeral head (A) treated with debridement using a curette to create a lesion (B) with well-defined vertical walls to facilitate more efficient load sharing with the normal surrounding articular cartilage (C).
Arthroscopic debridement of the glenohumeral joint is a palliative measure that appears to be useful for short-term pain relief in patients who present with coexisting pathology without gross mechanical symptoms coming from the defect. The senior author (B.J.C.) most commonly uses arthroscopic debridement and modification of the lesion’s transition zone for incidental lesions that do not seem to be categorically associated with the patient’s symptoms (Fig. 20–6).
 Reparative Techniques
Reparative Techniques
Microfracture
Marrow stimulation involves penetration of the subchondral bone by one of three methods: abrasion, drilling, and microfracture. Microfracture, the author’s preferred technique, was initially popularized by Steadman et al37,40 and has been widely applied to the knee. The technique recently described by us for the treatment of chondral defects of the knee is exactly what we perform in the shoulder.22,39 The basic steps include debridement through the calcified layer at the base of the defect, establishing vertical walls at the transition zone for reasons described previously, penetrating the base of the defect with a specialized awl with holes spaced 2 to 3 mm apart, and utilizing specific postoperative rehabilitation protocols.
The resultant tissue formed by the mesenchymal stem cells and growth factors is fibrocartilage, which is similar in appearance, but biomechanically inferior to hyaline cartilage.41,42 There are no clinical series of patients with focal chondral defects of the glenohumeral joint treated with microfracture alone. Siebold et al38 recently reported on five patients treated with a combination of microfracture and periosteal flap coverage at a mean follow-up of 25.8 months. Clinically, there were significant improvements in the Constant score and reductions in pain. Three patients underwent second-look arthroscopy at an average of 8 months, all with an improved appearance of the cartilage lesion.
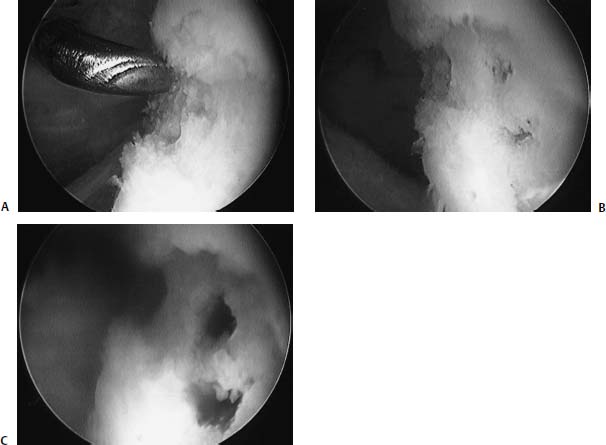
FIGURE 20–7 Arthroscopic photograph of an 18-year-old man with a focal chondral defect of the humeral head (A) treated with defect preparation and microfracture (B), leading to blood return emanating from the subchondral bone (C).
The senior author (B.J.C.) has performed microfracture of the glenohumeral joint in 10 patients with localized chondral defects of the glenohumeral joint (Fig. 20–7). The average age of the patients was 28. Sixty percent had humeral lesions, and 40% had glenoid lesions. Only two had kissing lesions. The average size of the lesion was 2.0 cm2. None of the patients had concomitant pathology. None were associated with instability. Postoperatively, these patients were treated with 4 to 6 weeks of pendulum exercises performed 600 times per day to mimic continuous passive motion. In all patients, there was a modest reduction in pain and improvements in function. However, no patients had complete elimination of their symptoms that were present preoperatively, and one patient is contemplating revision with a fresh osteochondral allograft transplant of the humeral head.
Microfracture of the glenohumeral joint is a measure that leads to fibrocartilage repair tissue. Although it may have some benefit in the knee, its usefulness in the shoulder is based on anecdotal implementation, with inferences that the same biologic milieu will occur in the glenohumeral joint that has been demonstrated in the knee joint. Nevertheless, the authors recommend microfracture as a first-line treatment of superficial defects believed to be associated with symptoms even in the setting where comorbidities are simultaneously corrected. Revision with restorative options is unlikely to be compromised following microfracture, and it can be performed entirely arthroscopically with very little morbidity. Osteochondral defects and mechanical symptoms are considered relative contraindications to microfracture, as these patients are unlikely to benefit. Practically speaking, for the reasons mentioned previously, smaller well-shouldered lesions should fare better clinically than larger unshouldered lesions with gradual transition zones.
 Restorative Techniques
Restorative Techniques
Osteochondral Autograft
Several authors have validated success with osteochondral autograft transplantation in the knee.43–45 Intuitively, patients with smaller lesions (i.e., less than 1–1.5 cm2) of the humerus who have demonstrated failure of first-line treatment, including microfracture, may be excellent candidates for this procedure. The advantages include the ability to restore the glenohumeral architecture with a viable “organ” of bone and cartilage with a single-stage procedure. It results in osseous integration and preserves the articular cartilage tidemark. Practically, it is easiest to harvest a donor plug from the lateral trochlea of the knee just proximal to the sulcus terminalis, but it is also possible through further research that a relatively noncontact donor site may be present in an adjacent area of the humeral articular surface. This could be accomplished arthroscopically, but defect location might also warrant a formal arthrotomy. Laszlo Hangody (personal communication, 2003) has performed this procedure in two patients, each with an osteochondral lesion of the posterior surface of the humerus. The grafts were harvested arthroscopically from the knee. In a unique application of osteochondral autograft transplantation, Connor et al46 reported on a patient with bilateral posterior fracture dislocations of the glenohumeral joint who was treated on one side with hemiarthroplasty, and on the contralateral side with a local autograft taken from the shoulder treated with arthroplasty, in a single-stage procedure where both shoulders where operated on simultaneously.
The use of osteochondral autografts as a restorative option is limited to patients with relatively small defects of the humerus who are willing to subject themselves to the potential complication of donor-site morbidity in an otherwise asymptomatic knee (Fig. 20–8). The best indication would be for patients with small osteochondral defects who complain of mechanical symptoms that would otherwise be poorly managed with palliative measures (i.e., microfracture). Fundamentally, osteochondral autografts represent an excellent solution, but the indications are narrow and are rarely present.
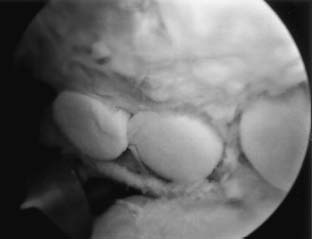
FIGURE 20–8 Arthroscopic photograph of an osteochondral autograft transplant performed for an isolated degenerative lesion of the humeral head. (Courtesy of Dr. John D. Kelly, IV, Department of Orthopedic Surgery, Temple University, Philadelphia.)
Fresh Osteochondral Allograft
Osteochondral allografting has been quite successful in the knee with the longest track record of any cartilage restoration procedure.47–49 Like osteochondral autografts, osteochondral allografts restore the congruency of the articular surface with an intact osteochondral segment. Graft preservation is critical. Contemporary treatment includes fresh (i.e., within 72 hours of donor asystole) or prolonged-fresh osteochondral grafts (i.e., ideally, maintained cold with regular medium change for no more than 28 days).50 The indications are for larger lesions (i.e., greater than 1.5–2.0 cm2) of the humeral head. As discussed earlier (see Decision Making), large uncontained lesions and osteochondral lesions in symptomatic patients are probably the best candidates. This is especially true for relatively older individuals who could also be considered as candidates for arthroplasty should the osteochondral allograft fail. There are only limited reports of the use of osteochondral allografts in the shoulder. In two case reports, fresh frozen humeral head allografts were used.51,52 In another, frozen or autoclaved femoral head grafts were used.53 Despite these limitations, very good outcomes were reported.
Osteochondral allografts are side- and size-matched using plain radiographs corrected for magnification. The technique is performed through a standard anterior deltopectoral approach. Technically, defects are treated using hand-fashioned grafts requiring supplemental fixation (Fig. 20–9). Newer instrumentation used in the knee (Arthrex, Inc., Naples, FL) in a technique recently described by the authors is another possibility that would potentially avoid the need for supplemental fixation.54 It is critical that the grafts are implanted with a minimal amount of subchondral bone that is irrigated copiously (i.e., no more than 6–8 mm) in an effort to minimize the immunogenic load contained in the osseous phase of the graft.

FIGURE 20–9 Case example of a large segmental defect of the proximal humerus treated by removing the fracture fragment and creating a wedge configuration of the recipient bed (A) and screw fixation of a fresh osteochondral allograft humeral head (B).
The use of osteochondral allografts as a restorative option is limited to patients with relatively large defects of the humerus who are willing to subject themselves to the infinitely low probability of the potential complication of donor disease transmission and the possibility of graft collapse. Osteochondral defects, such as those that occur with fracture-dislocations or engaging Hill-Sachs lesions, which lead to persistent instability despite standard stabilization techniques, are ideal lesions for this procedure. Because larger defects are more likely to become problematic and advances have occurred in terms of the availability of fresh osteochondral allograft transplant material, this solution represents an excellent first- or second-line option for appropriately selected patients.
Autogenous Chondrocyte Implantation
Autologous chondrocyte implantation (ACI) has a long track record for its implementation in the knee and the technique has been described by the senior author (B.J.C.) and others.55–62 The use of ACI in the shoulder is considered an off-label usage, and strict patient informed consent must be obtained. In brief, this procedure involves the harvesting of healthy articular cartilage with subsequent culturing and expansion of the cells over a 3- to 4-week period prior to implantation. Thus, it requires two procedures. The biopsy is obtained arthroscopically from the knee, and the implantation is performed through a standard deltopectoral approach. At the time of implantation, the periosteum is harvested from the proximal tibia in the standard location. As already mentioned,21 the articular cartilage of the humeral head is substantially thinner than that found in the knee, and thus suturing of the periosteum is technically far more challenging compared with our experience in the knee.
The optimal indications are for contained unipolar superficial defects of the humerus or glenoid in relatively young patients who have failed first-line treatment including microfracture. It is useful in larger lesions (>2 cm2) that are beyond the scope of osteochondral autograft transplantation and for superficial lesions where one would prefer to avoid violating the subchondral surface as is required of osteochondral allograft transplantation.
The authors recently published a case report of a young baseball player who had a defect develop in the anterosuperior humeral head following thermal capsulorrhaphy with a bipolar thermal device (Fig. 20–10).63 Cell-based technology has several advantages over osteochondral grafting. It utilizes the patient’s own tissue with minimal donor-site morbidity and does not violate the subchondral bone. The experience with this technology, however, is very limited and the indications are relatively small compared with some of the other technologies available.
Biologic Resurfacing
Unfortunately, a population of patients exists who have chondral lesions on both sides of the glenohumeral joint that ranges from localized to diffuse bipolar disease. The senior authors (B.J.C. and A.A.R.) have most commonly seen this in a population of patients referred following arthroscopic stabilization using suture anchors with and without the use of radiofrequency thermal capsulorraphy. These patients represent the most challenging treatment group as the treatment options are limited and relegated largely to anecdotal reports from surgeons interested in managing these problems with techniques considered investigational. Biologic resurfacing refers to solutions that provide some form of soft tissue interposition between the arthritic areas. This might include fascia lata, allograft tendon, the anterior shoulder capsule, periosteum, porcine small intestine submucosa or even allograft meniscus tissue.
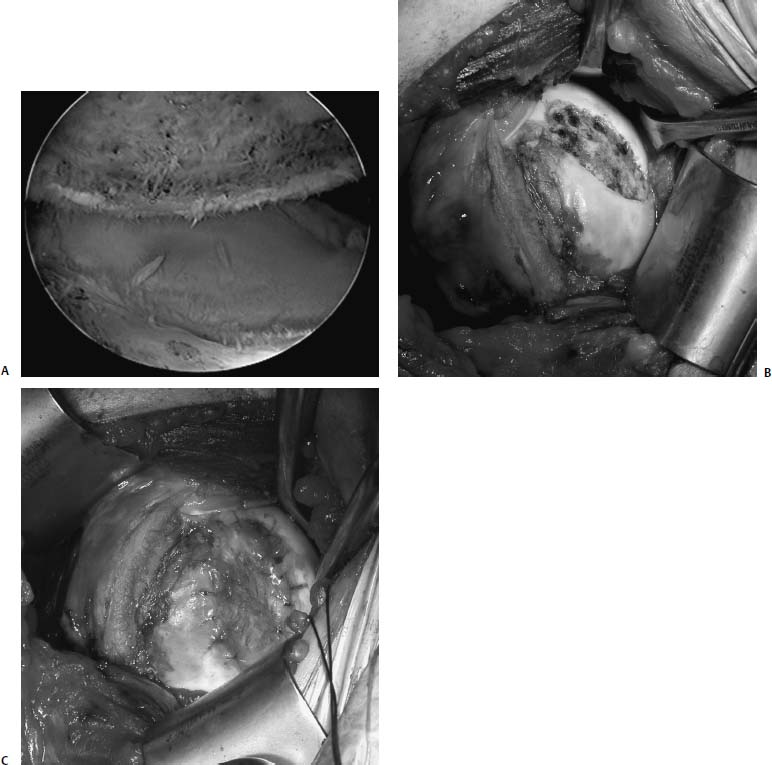
FIGURE 20–10 (A) Arthroscopic view of a focal chondral defect of the anterior humeral head in a 16-year-old boy treated previously with thermal capsulorrhaphy. (B) Intraoperative photograph showing the lesion covered by a thin layer of fibrous tissue. (C) Intraoperative photograph showing the periosteal patch sutured into place using 6–0 Vicryl suture and sealed with fibrin glue.
Burkhead and Hutton62 reported on the successful use of biologic resurfacing of the glenoid with fascia lata or anterior shoulder capsule in a relatively young population of patients who were treated with hemiarthroplasty in an effort to avoid glenoid replacement because of the significant risks for loosening in this at risk young population. Alternatives to this might include the use of porcine SIS (DePuy Orthopaedics, Inc., Warsaw, IN). Advantages of this material include the capability of inducing site-specific remodeling of various connective tissues.64,65 This application is most similar to the use of periosteum and microfracture discussed previously as reported by Siebold et al.38
Similar to ACI, the application of SIS in the shoulder is considered an off-label usage and explicit patient informed consent must be obtained. The senior author (B.J.C.) has a single case where SIS was used to resurface a microfractured glenoid in a girl who, for reasons unknown, developed extensive chondrolysis following arthroscopic shoulder stabilization using a monopolar radiofrequency device (Fig. 20–11). This patient had already failed attempts at debridement and capsular release. Early follow-up of 12 months demonstrated significant reductions in pain and improvements in function.
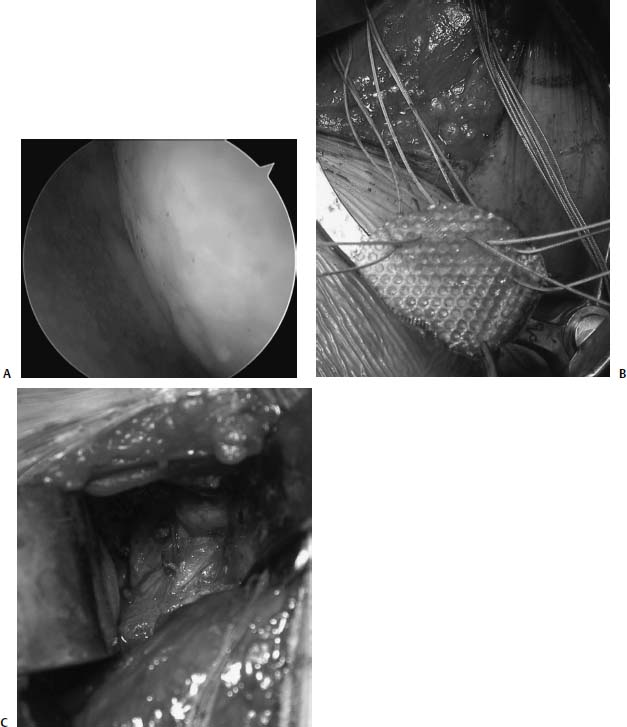
FIGURE 20–11 (A) Arthroscopic view of a 16-year-old girl treated previously with thermal capsulorrhaphy who demonstrated rapid progression of glenohumeral arthritis postoperatively. (B) Biologic resurfacing with small intestine submucosa (SIS) prepared to be sutured into place following microfracture of the glenoid. (C) SIS sewn into place.
Another option is to consider unloading the glenohumeral joint by placing an allograft meniscus along the periphery of the glenoid. This is the subject of a basic science study by the senior authors (B.J.C. and A.A.R.) as we have limited clinical experience with this technique (Fig. 20–12). Intuitively, glenoid lesions may respond favorably if reductions in contact area and force are achieved by suturing an allograft meniscus into the periphery of the glenoid. Similar to the use of SIS, this technique is considered investigational at this time, but offers a biomechanically attractive treatment alternative.
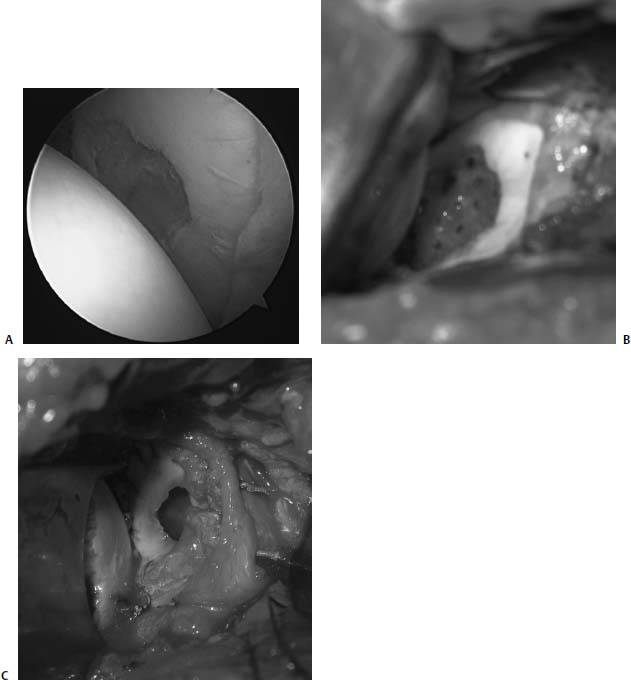
FIGURE 20–12 (A) Arthroscopic view of a 36-year-old man with a focal defect of the glenoid. (B) Defect exposed at the time of arthrotomy treated with a microfracture technique. (C) Allograft lateral meniscus sewn into place to the surrounding labrum.
A high degree of caution must exist when offering patients biologic resurfacing alternatives to reduce pain and improve function in the setting of chondral damage to the glenohumeral joint. Decisions must be made in the context of other comorbidities including range-of-motion loss, the integrity of the rotator cuff, and the stability of the shoulder. The indications for these techniques remain rare and are likely to evolve as our understanding of the biology and biomechanics of these treatment options improves.
 Postoperative Rehabilitation
Postoperative Rehabilitation
The rehabilitation following all of these techniques is critical to their success. Postoperatively early range of motion is important.66,67 Although we do not currently use continuous passive motion, patients begin with pendulum exercises the day after surgery with at least 600 cycles performed daily. Active range of motion is generally delayed until 6 weeks. Active range of motion and gentle strengthening begins at 6 to 8 weeks. Athletic endeavors are delayed until at least 6 months depending on the progress of the rehabilitation and the nature of the cartilage restoration procedure. The earliest time frame that patients can expect to get back to activities occurs following arthroscopic debridement. On the other hand, ACI and osteochondral allograft transplantation require the longest time frames postoperatively, and patients may not be permitted to engage in high-level activities for at least 12 months following their surgery.
 Conclusion
Conclusion
One has to individualize the treatment of each lesion once it is certain that the cartilage lesion is the source of the patient’s pain and impairment. A conservative approach is warranted given the lack of knowledge about the management of these defects. Beginning with palliative or reparative techniques may be the most prudent approach as patients may respond favorably and future treatment options are not compromised. If there is no or insufficient response to this initial treatment attempt, then one may consider a restorative option providing the level of the patient’s symptoms warrant further treatment. For smaller defects, osteochondral autografts may suffice. For larger lesions, ACI or fresh osteochondral allograft transplantation may be required. Biologic resurfacing must be approached with caution and on a case-by-case basis. Lessons learned from the implementation of cartilage procedures in the knee will assuredly improve upon our capabilities in the shoulder, but only through further objective investigation in the clinical and laboratory setting will we further our understanding of the treatment algorithm for the management of chondral injury in the glenohumeral joint.
REFERENCES
1. Fox JA, Kalsi RS, Cole BJ. Update on articular cartilage restoration. Tech Knee Surg 2003;2:2–17
2. Sellards RA, Nho SJ, Cole BJ. Chondral injuries. Curr Opin Rheumatol 2002;14:134–141
3. Cole BJ, Farr J. Putting it all together. Orthop Tech 2001;11: 151–154
4. Miller M, Cole BJ. Atlas on chondral injury. Orthop Tech 2001;11: 145–150
5. Outerbridge GETRE. The etiology of chondromalacia patellae.J Bone Joint Surg Br 1961;43:742–757
6. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy 1997;13:456–460
7. Hjelle K, Solheim E, Torbjorn S, Rune M, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy 2002;18:730–734
8. Guntern DV, Pfirrmann CW, Schmid MR, et al. Articular cartilage lesions of the glenohumeral joint: diagnostic effectiveness of MR arthrography and prevalence in patients with subacromial impingement syndrome. Radiology 2003;226: 165–170
9. Hsu HC, Luo ZP, Stone JJ, Huang TH, An KN. Correlation between rotator cuff tear and glenohumeral degeneration. Acta Orthop Scand 2003;74:89–94
10. Gartsman GM, Taverna E. The incidence of glenohumeral joint abnormalities associated with full-thickness, reparable rotator cuff tears. Arthroscopy 1997;13:450–455
11. Werner A, Lichtenberg S, Nikolic A, Habermeyer P. Intraarticular pathology of atraumatic shoulder dislocations: an arthroscopic study. Unfallchirurg 2003;106:110–113
12. Taylor DC, Arciero RA. Pathologic changes associated with shoulder dislocations. Arthroscopic and physical examination findings in first-time, traumatic anterior dislocations. Am J Sports Med 1997;25:306–311
13. Cameron ML, Kocher MS, Briggs KK, Horan MP, Hawkins RJ. The prevalence of glenohumeral osteoarthritis in unstable shoulders. Am J Sports Med 2003;31:53–55
14. Hintermann B, Gachter A: Arthroscopic findings after shoulder dislocation. Am J Sports Med 1995;23:545–551
15. Carroll KW, Helms CA, Speer KP. Focal articular cartilage lesions of the superior humeral head: MR imaging findings in seven patients. AJR Am J Roentgenol 2001;176:393–397
16. Cameron BD, Galatz LM, Ramsey ML, et al. Non-prosthetic management of grade IV osteochondral lesions of the glenohumeral joint J Shoulder Elbow Surg 2002;11:25–32
17. Chung CB, Frank LR, Resnick D. Cartilage imaging techniques: current clinical applications and state of the art imaging. Clin Orthop 2001;391(suppl):S370–S378
18. Karantanas AH, Zibis AH, Kitsoulis P. Fat-suppressed 3D-T1-weighted-echo planar imaging: comparison with fat-suppressed 3D-T1-weighted-gradient echo in imaging the cartilage of the knee. Comput Med Imaging Graph 2002;6:159–165
19. Gold GE, Beaulieu CF. Future of MR imaging of articular cartilage. Semin Musculoskelet Radiol 2001;5:313–327
20. Matsui N, Kobayashi M. Application of MR imaging for internal derangement of the knee (orthopedic surgeon view). Semin Musculoskel Radiol 2001;5:139–141
21. Glenn RE, Cole BJ, Fox JA, Meininger AK, Romeo AA, Hayden JK. Articular cartilage thickness of the humeral head: an anatomic study. Review, Corr, September, 2005.
22. Fox J, Cole BJ. Update on articular cartilage restoration. Tech Knee Surg 2003;2:2–17
23. Ishikawa H, Ueba Y, Yonezawa T, Kurosaka M, Ohno O, Hirohata K. Osteochondritis dissecans of the shoulder in a tennis player. Am J Sports Med 1988;16:547–550
24. Pydisetty RV, Prasad SS, Kaye JC. Osteochondritis dissecans of the humeral head in an amateur boxer. J Shoulder Elbow Surg 2002;11:630–632
25. Shanley DJ, Mulligan ME. Osteochondrosis dissecans of the glenoid. Skeletal Radiol 1990;19:419–421
26. Hamada S, Hamada M, Nishiue S, Doi T. Osteochondritis dissecans of the humeral head. Arthroscopy 1992;8:132–137
27. Anderson WJ, Guilford BW. Osteochondritis dissecans of the humeral head. An unusual cause of shoulder pain. Clin Orthop 1983;173:166–168
28. Ganter M, Reichelt A. Osteochondrosis dissecans of the humeral head. Z Orthop Ihre Grenzgeb 1996;134:73–75
29. Rudd RG, Visco DM, Kincaid SA, Cantwell HD. The effects of beveling the margins of articular cartilage defects in immature dogs. Vet Surg 1987;16:378–383
30. Ellman H, Harris E, Kay SP. Early degenerative joint disease simulating impingement syndrome: arthroscopic findings. Arthroscopy 1992;8:482–487
31. Witwity T, Uhlmann R, Nagy MN, et al. Shoulder rheumatoid arthritis associated with chondromatosis, treated by arthroscopy Arthroscopy 1991;7:233–236
32. Lu Y, Edwards RB III, Cole BJ, Markel MD. Thermal chondroplasty with radiofrequency energy. An in vitro comparison of bipolar and monopolar radiofrequency devices. Am J Sports Med 2001;29: 42–49
33. Kelly E, O’Driscoll S, Steinmann S. Arthroscopic glenoidplasty and osteocapsular arthroplasty for advanced glenohumeral arthritis. Presented at the annual open meeting of the American Shoulder and Elbow Surgeons, 2001, San Francisco, CA.
34. Matthews LS, LaBudde JK. Arthroscopic treatment of synovial diseases of the shoulder Orthop Clin North Am 1993;24: 101–109
35. Midorikawa K, Hara M, Emoto G, et al. Arthroscopic debridement for dialysis shoulders Arthroscopy 2001;17:685–693
36. Weinstein DM, Bucchieri JS, Pollock RG, et al. Arthroscopic debridement of the shoulder for osteoarthritis. Arthroscopy 2000;16:471–476
37. Steadman JR, Briggs KK, Rodrigo JJ, et al. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy 2003;19:477–484
38. Siebold R, Lichtenberg S, Habermeyer P. Combination of microfracture and periosteal flap for the treatment of focal full-thickness articular cartilage lesions of the shoulder: a prospective study. Knee Surg Sports Traumatol Arthrosc 2003;11:183–189
39. Freedman JB, Coleman SH, Olenac C, Cole BJ. The biology of articular cartilage injury and the microfracture technique for the treatment of articular cartilage lesions. Semin Arthroplasty 2002;13:202–209
40. Steadman JRR, WG, Singleton SB, Briggs KK. Microfracture technique for full-thickness chondral defects: technique and clinical results. Oper Tech Orthop 1997;7:300–304
41. Buckwalter JA, Mow VC. Cartilage repair in osteoarthritis. In: Moskowitz RW, Howell DS, Goldberg VM, Mankin HJ, eds. Osteoarthritis: Diagnosis and Management. Philadelphia: W.B. Saunders, 1992:71–107
42. Athanasiou KA, Rosenwasser MP, Spiker RL, Mow VC. Effects of passive motion on the material properties of healing articular cartilage. Trans Orthop Res Soc 1991;15:156
43. Hangody L, Feczki P, Bartha L, et al. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop 2001;391(suppl):S328–S336
44. Kish G, Modis L, Hangody L. Osteochondral mosaicplasty for the treatment of focal chondral and osteochondral lesions of the knee and talus in the athlete. Rationale, indications, techniques, and results. Clin Sports Med 1999;18:45–66
45. Hangody L, Kish G, Karpati Z, et al. Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice. Orthopedics 1998;21:751–756
46. Connor PM, Boatright JR, D’Alessandro DF. Posterior fracture-dislocation of the shoulder: treatment with acute osteochondral grafting. J Shoulder Elbow Surg 1997;6:480–485
47. Bugbee WD. Fresh osteochondral allografting. Oper Tech Sports Med 2000;8:158–162
48. Gross AE, Aubin P, Cheah HK, Davis AM, Ghazavi MT. A fresh osteochondral allograft alternative. J Arthroplasty 2002; 17(4, suppl 1) 50–53
49. Gross AE. Fresh osteochondral allografts for post-traumatic knee defects: surgical technique. Oper Tech Orthop 1997;7:334–339
50. Williams JM, Virdi AS, Pylawka TK, Edwards RB 3rd, Markel MD, Cole BJ. Prolonged-fresh preservation of intact whole eanine femoral condyles for the potential use as osteochondral allografts. J Ortho Res 2005;23:831–837
51. Johnson DL, Warner JJ. Osteochondritis dissecans of the humeral head: treatment with a matched osteochondral allograft. J Shoulder Elbow Surg 1997;6:160–163
52. Yagishita K, Thomas BJ. Use of allograft for large Hill-Sachs lesion associated with anterior glenohumeral dislocation. A case report. Injury 2002;33:791–794
53. Gerber C, Lambert SM. Allograft reconstruction of segmental defects of the humeral head for the treatment of chronic locked posterior dislocation of the shoulder. J Bone Joint Surg 1996;78:376–382
54. Fox JA, Freedman KB, Lee JL, Cole BJ. Fresh osteochondral allograft transplantation for articular cartilage defects. Oper Tech Sports Med 2002;10:168–173
55. D’Amato M, Cole BJ. Autologous chondrocyte implantation. Orthop Tech 2001;11:115–131
56. Peterson L, Brittberg M, Kiviranta I, et al. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med 2002;30:2–12
57. Minas T. Autologous chondrocyte implantation for focal chondral defects of the knee. Clin Orthop 2001;391:S349–S361
58. Micheli LJ, Browne JE, Erggelet C, et al. Autologous chondrocyte implantation of the knee: multicenter experience and minimum 3-year follow-up. Clin J Sports Med 2001;11:223–228
59. Peterson L, Minas T, Brittberg M, et al. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop 2000;374:212–234
60. Gillogly SD, Voight M, Blackburn T. Treatment of articular cartilage defects of the knee with autologous chondrocyte implantation. J Orthop Sports Phys Ther 1998;28:241–251
61. Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331:889–895
62. Burkhead WZ, Hutton KS. Biologic resurfacing of the glenoid with hemiarthroplasty of the shoulder. J Shoulder Elbow Surg 1995;4:263–270
63. Romeo AA, Cole BJ, Mazzocca AD, Fox JA, Freeman KB, Joy E. Autologous chondrocyte repair of an articular defect in the humeral head. Arthroscopy 2002;18:925–929
64. Badylak SF, Tullius R, Kokini K, et al. The use of xenogeneic small intestinal submucosa as a biomaterial for Achilles tendon repair in a dog model. J Biomed Mater Res 1995;29:977–985
65. Dejardin LM, Arnoczky SP, Clarke RB. Use of small intestinal submucosal implants for regeneration of large fascial defects. An experimental study in dogs. J Biomed Mater Res 1999;46: 203–211
66. Salter RB, Bell RS, Keeley FW. The protective effect of continuous passive motion in living articular cartilage in acute septic arthritis: an experimental investigation in the rabbit. Clin Orthop 1981;159:223–247
67. Williams JM, Moran M, Thonar EJ, Salter RB. Continuous passive motion stimulates repair of rabbit knee articular cartilage after matrix proteoglycan loss. Clin Orthop 1994;304:252–222
< div class='tao-gold-member'>



