INTRODUCTION
Cancer is the uncontrolled growth of cells, which damages healthy tissue and causes disease. The American Cancer Society differentiates more than 100 types of cancers that manifest in diverse ways throughout the human body. Treatment of cancer can vary from local to systemic and from mildly invasive to radical depending on the type of cancer and the extent of disease.
With nearly 12 million cancer survivors in the United States, cancer rehabilitation is a growing field. The goal of this chapter is to highlight the most common issues in the care of these patients that are pertinent to physiatrists. Physiatrists are experts in restoring function and improving quality of life for patients, making them well trained to address the rehabilitative needs of the growing cancer survivor population. As in other settings for rehabilitation, a multidisciplinary team approach is ideal throughout the diagnosis, treatment, survivorship, and palliative care of patients.
OVERVIEW OF CANCER REHABILITATION
More than 1.6 million new cases of cancer were diagnosed in the United States in 2012. With advancements in health care, people are living longer, and as they age, their risk of developing cancer increases. The greatest incidence of cancer is between the ages of 65 and 74 years. The three most common cancers diagnosed in the United States are those of the prostate, female breast, and lung; these unfortunately also have the highest mortality rates.
Cancer rehabilitation was first documented by Drs Howard Rusk and Eugene Taylor in 1949. Funding for cancer rehabilitation was established in 1965 by the Rehabilitation Act, which provided 75% of cost by federal dollars. In 1973 the National Rehabilitation Act provided protection from discrimination for people with handicaps, now defined as disabilities; this was the precursor to the Americans with Disabilities Act, enacted in 1990. More recently, the requirements for the American College of Surgeons’ Commission on Cancer accreditation state that hospitals and cancer centers must provide rehabilitation services to cancer survivors either at their primary facility or by referral.
Two programs that were in the forefront of cancer rehabilitation in the 1960s have maintained their status as leaders in the field of cancer rehabilitation today. They are the University of Texas MD Anderson Cancer Center and a cooperative program with Drs Howard Rusk and J Herbert Dietz, in New York, that has become Memorial Sloan Kettering Cancer Center. These two centers are the only locations in the United States that offer a fellowship dedicated to training physicians in cancer rehabilitation.
Dietz created the first definition of cancer rehabilitation derived from evidence-based medicine. He also created a classification system for the goals of rehabilitation therapy, differentiating among four phases of care: prevention, restoration, supportive care, and palliative care. Prevention is treatment provided to a patient before development of a potential disability that is expected to lessen the severity of disability or its duration. Restoration is the return of the patient to a premorbid state without handicap or known residual disease, including return to gainful occupation. Supportive care is control of ongoing disease while the patient remains active and productive but with known residual disease and possibly a slowly progressive handicap. In this stage, increased tolerance and circumvention of the residual disability can be expected from adequate supportive training and care. Palliative care addresses increasing disability expected from relentless progression of disease, with a focus on provision of an appropriate program that will prevent or reduce complications that might otherwise develop. These complications include, but are not limited to, bedsores, pain, contractures, problems with personal hygiene, weakness, and emotional deterioration secondary to inactivity and depression.
The number of cancer survivors in the United States has increased more than threefold in the past 30 years. With nearly two thirds of newly diagnosed cancer patients expected to survive at least 5 years or more, it is essential for physiatrists to understand the disease processes, treatment options, and complications that confront cancer survivors.
CANCER-RELATED FATIGUE
Cancer-related fatigue (CRF) is a highly prevalent and distressing symptom affecting cancer patients. The National Comprehensive Cancer Network (NCCN) defines CRF as a “distressing, persistent, subjective sense of physical, emotional and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.” Clinical features include loss of interest; exhaustion; lack of energy; impaired sleep, memory, and cognition; pain; anxiety; and depression. Thus, CRF can cause further physical, psychological, emotional, and economic sequelae for the patient, caregivers, and family. Estimates of prevalence range from 50–90%. It may occur at any time during the disease state from diagnosis through treatment, and including the years following treatment. To adequately treat CRF, underlying mechanisms must be elucidated.
The pathophysiology of CRF is complicated, multifactorial, and not well understood. It remains uncertain whether CRF is a result of the tumor itself, treatment of the tumor, behavioral or environmental factors, or has a genetic disposition. Proposed pathophysiologic factors include dysregulation of inflammatory cytokines, gene polymorphisms, changes in the central nervous system (CNS) serotoninergic system, disturbances of the hypothalamic regulatory circuit, and disturbances of circadian melatonin secretion. Factors that may exacerbate or precipitate CRF include pain, nutritional status, anemia, deconditioning, sleep disturbances, and existing comorbidities.
Several theories have been proposed to explain the mechanism of CRF. Dysregulation of the serotonin system is often implicated in the pathogenesis of CRF. Serotonin (5-HT) has many functions, including control of appetite, sleep, memory, mood, behavior, cardiovascular function, endocrine regulation, and depression. It is believed that 5-HT levels or 5-HT receptors may be upregulated in patients with cancer, causing an overall decrease in somatomotor drive. The metabolism of serotonin may be affected by any of the proinflammatory cytokines, specifically tumor necrosis factor-alpha (TNF-α) and cytokines such as interleukin-1b.
The hypothalamic–pituitary–adrenal axis is another pathway affecting fatigue. Theories that focus on this mechanism propose that any cancer treatment or the cancer itself may affect the axis system, thus causing endocrine changes contributing to fatigue. Cortisol and corticotropin-releasing hormone appear to be implicated in this pathway.
Just as the causes of CRF are multifactorial, so are treatment interventions. The NCCN identifies four categories of CRF intervention: education and counseling, management of fatigue, nonpharmacologic measures, and pharmacologic measures. Treatment should be comprehensive to include all categories, and should begin as early as time of diagnosis. Energy conservation techniques help to manage fatigue, and strengthening helps to alleviate deconditioning and inactivity. Exercise is known to improve functional capacity, thereby reducing energy expenditure for activities of daily living. Moderate aerobic exercise seems to provide the most benefit. Additional nonpharmacologic interventions include stress management, yoga, acupuncture, mindfulness-based stress reduction, and cognitive-behavioral therapy. Pharmacologic interventions include treatment of anemia, psychostimulants, and proper pain management. Methylphenidate and modafinil are recognized to improve fatigue. Use of corticosteroids has also been studied in the treatment of CRF.
CANCER PAIN
Pain in the cancer patient is a challenge to both patient and provider. It is estimated to affect between 30% and 50% of those actively undergoing treatment, and upwards of 70% of those with advanced disease, thus having major implications on quality of life. While pain may be the first diagnostic sign of malignancy, it may also be present at any time, and the frequency and intensity of pain increase with advanced stages of cancer.
Pain may be divided into three categories: somatic, visceral, and neuropathic. The first two of these are considered nociceptive. Pain may be caused by tumor infiltration or occur as a result of additional treatments (eg, radiation, surgery, or chemotherapy). Examples of peripheral neuropathic pain include radiation neuropathy, chemotherapy-associated neuropathy, surgery-related nerve injury, and plexopathy.
Primary afferent sensory neurons are the pathway by which sensory information from the periphery is referred to the spinal cord and brain. The cell bodies of sensory fibers are located in the trigeminal and dorsal root ganglion, consisting of large-diameter myelinated Aβ fibers and small-diameter thinly myelinated Aδ and unmyelinated C fibers. It is the smaller diameter C fibers and Aδ fibers—known as nociceptors—that generate chronic pain in cancer patients.
Once a malignancy has caused local or systemic tissue injury, neurotransmission of these nociceptors is altered. Hyperalgesia, the perception of mildly noxious stimuli as highly noxious, and allodynia, the perception of normally nonnoxious sensory information as noxious, alter the body’s normal pain interpretation. This pathway plays a role in both somatic as well as visceral pain.
Visceral pain is described as diffuse and poorly localized; it is often referred to other locations and may be accompanied by motor and autonomic reflexes. It is typically described as dull, aching, deep, vague, or diffuse. In contrast, neuropathic pain is commonly described as burning, shooting, or lancinating. Associated negative motor phenomena (weakness, fatigue) or positive motor phenomena (tremors, ataxia, dystonia, and dyskinesia) may occur with neuropathic pain. Pain sensitization may occur peripherally, centrally, or through both routes, which adds to the complexity of treatment.
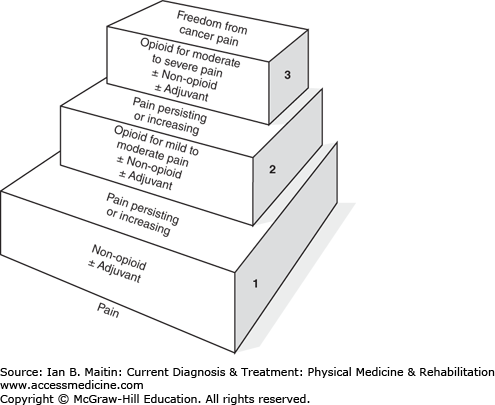
Once the type of pain has been diagnosed, management is paramount. Options include pharmacologic as well as nonpharmacologic measures and should be used in combination, as this has been established by the World Health Organization (WHO) as the standard of care. The WHO developed the pain control ladder (Figure 35–1), a stepwise approach to initiation of analgesic control. For mild pain, nonopioids with or without an adjuvant are recommended. Adjuvant medications include nonsteroidal antiinflammatory drugs, topical analgesics, antidepressants, anticonvulsants, corticosteroids, and anxiolytics. Additional agents such as pregabalin have also been shown to be efficacious in treating neuropathic cancer pain. Once a moderate level of pain is reached, opioids may be introduced. To maintain adequate pain control, analgesics should be given around the clock every 3–6 hours rather than as needed.
For patients with severe, refractory pain or chronic pain, interventional techniques offer another treatment option. Neurolysis, trigger point injections, sympathetic blocks, vertebroplasty, and spinal cord stimulation are examples of available interventional options as adjuncts to pharmacologic therapy to improve neuropathic pain.
Other nonpharmacologic options exist and have been studied in the cancer patient. Modalities such as exercise, yoga, acupuncture, use of transcutaneous electrical nerve stimulation (TENS) units, biofeedback, and psychosocial interventions all have been shown to be efficacious for the management of cancer pain. In addition to ameliorating pain, exercise and yoga may also reduce fatigue, thereby improving quality of life. Bracing and orthotics may help to offload joints, provide stability, and help with weakness thereby decreasing pain.
RADIATION TOXICITY
Approximately 50% of all patients diagnosed with cancer will require radiation therapy at some point during the course of their disease. Radiation can be used with intent to cure, or palliatively to control pain, prolong life, or preserve function. Radiation therapy can be delivered by means of external beams (external beam radiation therapy) or radioactive material placed internally (brachytherapy). The dose of radiation is determined by tissue tolerance (radiosensitivity) of the target tissue; Table 35–1 lists examples of organ-specific tolerance doses. The therapeutic intent of radiation is to kill fast-dividing cancer cells while sparing the relatively slower growing somatic cells. Patient-related factors, including cancer size, location, and tissue radiosensitivity, play an integral role in dosing. Patient-related factors that are incorporated into the treatment plan include age, obesity, prior surgery, trauma, and the presence of microvascular diseases such as diabetes, hypertension, or collagen vascular disease.
| Organ | Single Dose (Gy) | Fractionated Dose (Gy) |
|---|---|---|
| Brain | 15–25 | 60–70 |
| Eye (lens) | 2–10 | 6–12 |
| Skin | 15–20 | 30–40 |
| Spinal cord | 15–20 | 50–60 |
| Vasculoconnective tissue system | 10–20 | 50–60 |
| Mucosa | 5–20 | 65–77 |
| Peripheral nerve | 15–20 | 65–77 |
| Muscle | > 30 | > 70 |
| Bone and cartilage | > 30 | > 70 |
| Thyroid | — | 30–40 |
Radiation doses are currently measured in grays (Gy) or centigrays (cGy). Radiation was previously measured in rads (1 Gy = 100 cGy = 100 rads). Fraction refers to the amount of radiation delivered in one treatment session. Total dose radiation takes into account the fractions repeated over a period of time and boosts the dose if it was delivered. Hyperfractionated regimens deliver smaller radiation doses more than once a day and therefore decrease associated radiation complications. Hypofractionated radiation regimens deliver higher radiation doses in fewer treatment sessions. Dose-sculpting techniques such as image-guided radiation therapy (IGRT) and intensity-modulated radiation therapy (IMRT) allow for very tight confirmation of the radiation beam so that tumors near vital structures (eg, the spinal cord) can be radiated to within a few millimeters. Such techniques allow for radiation to be given in a single fraction in certain instances, which allows tumors that were previously thought radioresistant (eg, melanoma) to be effectively treated.
The complications associated with radiation toxicity are numerous and can be categorized as acute and late effects (Table 35–2). Acute effects occur during treatment or shortly afterward, while late effects occur months to years later. Acute complications damage rapidly proliferating cells; therefore, the effects are typically temporary and often resolve during the course of treatment. Late effects can appear decades after treatment and progress for the duration of the patient’s life. There is a risk for secondary carcinomas as well as toxic effects on organs within the radiation field, including cardiomyopathies, pulmonary fibrosis, and thyroid dysfunction. Approximately 10% of people who receive radiotherapy ultimately develop a secondary cancer. Children treated before the age of 15 years are at the highest risk.
| Acute Effects | Late Effects |
|---|---|
Fatigue Nausea Vomiting Anorexia Desquamation Dermatitis Mucositis Xerostomia Taste loss Proctitis Cystitis Decreased libido Sterility Amenorrhea Hematologic changes Pneumonitis Diarrhea Esophagitis Conjunctivitis Infection Epilation or alopecia | Soft-tissue fibrosis Skin atrophy Auditory changes Pulmonary fibrosis Gastrointestinal stricture Thyroid dysfunction Brain necrosis Myelitis Plexopathy Lymphedema Secondary malignancies Osteonecrosis Telangiectasias Incontinence Diarrhea |
RADIATION FIBROSIS SYNDROME
Radiation has damaging effects on multiple structures, including soft tissue, ligament, muscle, nerve, blood vessels, and the lymphatic system. The term radiation fibrosis syndrome is used to describe the numerous neuromuscular, musculoskeletal, and organ complications that occur as a direct result of radiation-induced fibrosis. It is a common phenomenon; approximately 60% of all patients receiving radiation therapy eventually develop radiation-induced fibrosis.
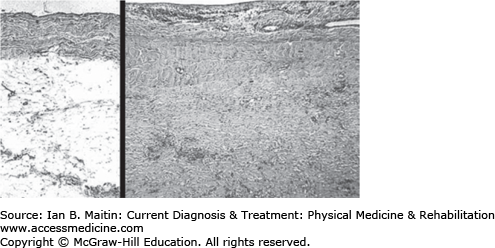
Microvascular injury appears to be the critical feature in both acute and chronic radiation toxicity of normal tissue. The pathologic effect of radiation is the formation of a rigid interstitial fibrinous exudate (fibrin) preceding progressive fibrotic encasement of blood vessels, thus distorting surrounding tissue (Figure 35–2). The effects of radiation can cause injury to the nervous system at every level, including encephalopathy, myelopathy, radiculopathy, plexopathy and mononeuropathies, and myopathy. Nerve damage may be secondary to compromise of the vasa nervorum, the vascular supply of the nervous tissue.
Figure 35–2
Radiation-induced fibrosis. Sections of parietal pericardium at equal magnifications. Left: Normal pericardium showing an (upper) layer of fibrous tissue and a (lower) thick layer of adipose tissue. Right: Extensive fibrosis of irradiated pericardium replacing adipose tissue. Hematoxylin and eosin stain. (Reproduced with permission from Luis F Fajardo, MD, Stanford Medical School.)
Acute radiation encephalopathy can occur with a single fraction greater than 300 Gy due to increased intracranial pressure from whole-brain radiation therapy. Symptoms include somnolence, headache, and progressive focal neurologic deficits. The combination of imaging, laboratory studies, and clinical presentation can distinguish encephalopathy from recurrence or infection. It is typically self-limiting, and corticosteroids may be utilized to decrease edema. Chronic radiation encephalopathy associated with cerebral atrophy manifests with dementia, cognitive deficits, ataxia, and urinary incontinence. Encephalopathy from radiation necrosis typically occurs 1–2 years after treatment, with a 3–5% occurrence rate, and is associated with doses greater than 5000 Gy. It can be difficult to distinguish necrosis from tumor recurrence by magnetic resonance imaging (MRI); hence, positron emission tomography (PET) is the study of choice. Treatment is supportive, consisting of corticosteroids or resection. Surgical decompression may not reverse the functional decline in the majority of patients and serves as more of a palliative measure. Bevacizumab has shown promising results clinically and radiologically for reversal of cerebral radionecrosis.
Myelopathy is associated with radiation levels in excess of 5000 Gy, with an average of 14 months latency. Presenting symptoms are typically sensory abnormalities in the lower extremities with ascending weakness up to the site of radiation or a Brown-Séquard syndrome presentation. Hyperreflexia with Babinski’s and Lhermitte’s signs are commonly present. Painful paresthesias may be experienced in the dermatomal distribution correlating with the spinal level of irradiation.
Radiation-induced brachial plexopathies occur more commonly than lumbosacral plexopathies and are associated with fractions to the brachial plexus greater than 2 Gy. Clinically, radiation-induced plexopathy is less likely to be painful than infiltrative neoplastic plexopathy and is more likely to involve the upper trunk. Diagnosis is made based on imaging studies, and the finding of myokymia on electrodiagnostic study is highly suggestive of a radiation-induced cause but does not exclude tumor. Peripheral neuropathies are less common and occur with cumulative doses greater than 6000 Gy but can be seen with lower doses. The phrenic and recurrent laryngeal nerves can be affected, and patients present with dysphagia, hoarseness, and respiratory distress. Radiation fibrosis management has been attempted with pentoxifylline, superoxide dismutase, hyperbaric oxygen therapy, and a combination of pentoxifylline and tocopherol, with mixed results.
NERVOUS SYSTEM CHEMOTHERAPY-INDUCED TOXICITY
Cancer patients experience numerous side effects from both the disease and the antineoplastic agents used to treat it. The CNS and peripheral nervous system (PNS) are vulnerable targets of chemotherapy agents; however, the CNS is less vulnerable than the PNS to toxic exogenous agents owing to the blood–brain barrier. Therefore, with lower dose antineoplastic agents, changes will be seen in the PNS before the CNS.