Burn Injury
Frederick W. Endorf
Saman Arbabi
Approximately 500,000 Americans suffer burn injuries each year, with approximately 40,000 requiring hospital admission for treatment of their burns. It is estimated that 4,000 people will die annually in the United States as a result of burns.1 A proper understanding of the diagnosis and treatment of burn injuries is essential for trauma and critical care surgeons.
HISTORY
Burn care has undergone a remarkable evolution over the course of the last century. In the early 1900s, burn patients typically faced an extremely grim prognosis. If one third of the body surface area was involved, even with superficial burns, death was considered inevitable.2 Great strides were made in understanding burn resuscitation following major disasters such as the 1930 Rialto Concert Hall,3 1942 Cocoanut Grove,4 and 1947 Texas City fires.5 The advent of early excision of the burn wound in the 1970s decreased burn wound sepsis and shortened hospital stays.6,7 Subsequent improvements in critical care and wound management have decreased mortality to the point that survival is often expected. Long-term functional outcomes have instead become the goals of burn care. The American College of Surgeons (ACS), along with the American Burn Association (ABA), realizing that burn care is a specialized and constantly changing field, initiated a burn center verification process to ensure that high standards are met in the care of burn patients. Participating burn centers are verified every 3 years with the goal of providing reliable, excellent care to burn patients.8 Burn care has evolved into a multidisciplinary effort that sets it apart from many other subspecialties, with attention to psychological and rehabilitation issues along with traditional medical and surgical care. The wideranging nature of this comprehensive care exceeds the scope of this chapter, and here we focus primarily on the surgical and critical care topics germane to the care of burn patients.
MECHANISMS OF BURN INJURY
Burns maybe caused by anynumber of agents, butare most commonly grouped into thermal, electrical, and chemical burns. Thermal burns are the most common, including fire or flame burns, scalds, and contact burns. Of patients admitted to burn centers, 46% have fire or flame injuries, 32% scalds, and 8% contact burns from hot objects. Of the 4,000 burn-related deaths each year, approximately 3,500 are linked with residential fires, and often are a result of inhalation injury and carbon monoxide (CO) poisoning.1 Electrical injuries are less common (4% of burn admissions)1 but can be severe. Special considerations in the care of electrical injury include the potential for cardiac arrhythmias, extremity compartment syndromes, and rhabdomyolysis. An electrocardiogram (ECG) is recommended in all patients with electrical injury, but low-voltage injuries without ECG abnormalities or other injuries do not necessarily require hospital admission.9 A high index of suspicion should be maintained for compartment syndrome with concurrent rhabdomyolysis, and fasciotomies should be performed as indicated by neurologic or vascular compromise. Examination of vision and hearing is also important, particularly in lightning injuries.10 Chemical burns are also less common, but can also cause severe burns. Attention should be given to complete removal of the substance from the patient through copious irrigation of the affected area for at least 30 minutes. One must also look for metabolic insults caused by the offending agent, such as acidosis, hemolysis, and hemoglobinuria with formic acid or hypocalcemia with hydrofluoric acid.11 Hydrofluoric acid is a common culprit due to its inclusion
in industrial cleansers. Treatment is neutralization with calcium, through calcium gluconate in dimethyl sulfoxide applied directly to wounds, or subcutaneous or intravenous infiltration of calcium gluconate.12,13 Intra-arterial infusion of calcium gluconate has been reported in facial hydrofluoric acid burns.14
in industrial cleansers. Treatment is neutralization with calcium, through calcium gluconate in dimethyl sulfoxide applied directly to wounds, or subcutaneous or intravenous infiltration of calcium gluconate.12,13 Intra-arterial infusion of calcium gluconate has been reported in facial hydrofluoric acid burns.14
BURN DEPTH
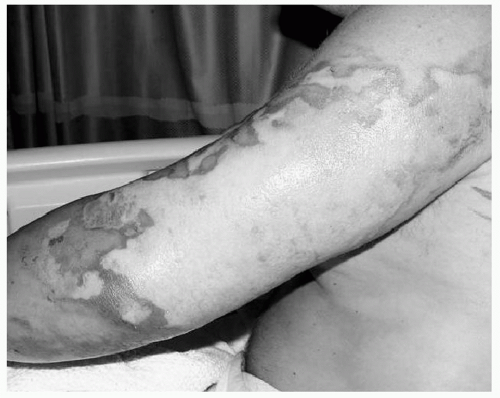
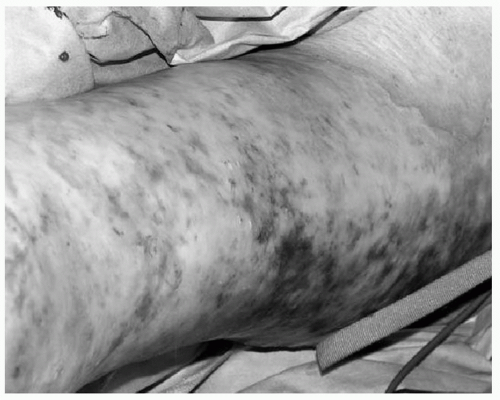
Traditionally, burn depth was classified using first, second, and third degrees. This system has gradually been replaced with the terms superficial, superficial partial thickness, deep partial thickness, and full thickness. Superficial burns are typically caused by ultraviolet light (i.e., “sunburn”) or by very short flash burns. These burns are dry and red, blanch with pressure, are painful to touch, and heal in 3 to 6 days without scarring. Partial-thickness burns may be further divided into superficial and deep depending on the depth of dermis involved. Superficial partial-thickness burns are often caused by splash scalds or short flash burns. They will blister, and are red and weeping and blanch with pressure (see Fig. 1). These burns are painful to touch, air and temperature, and most will heal without surgery after 7 to 20 days. Scarring is uncommon, although pigment changes in the skin may be seen. Deep partial-thickness burns may be caused by scalds, flame burns, or oil or grease burns. These burns also blister, but can be wet or have a waxy dry texture, with a patchy red or white appearance. Deep partial-thickness burns are typically insensate. Surgery is usually required because these burns take more than 3 weeks to heal, and are especially prone to infection, hypertrophic scarring, and contractures if allowed to heal without excision and grafting. Full-thickness burns are white, gray, or black. They are insensate and do not blanch with pressure (see Fig. 2). Any full-thickness burns >2% of the total body surface area (TBSA) are not likely to heal, and even small full-thickness burns have severe risks of scarring and contractures.15
Assessment of burn depth is important because the vast majority of superficial partial-thickness burns will heal without surgery, but most deep partial-thickness burns require excision and skin grafting. Even the most adept burn surgeons can be fooled when predicting whether partial-thickness burns will heal, and several areas of research have been devoted to improving estimations of burn depth. Histologic diagnosis by full-thickness skin biopsy gives a consistently accurate measure of burn depth. Unfortunately, pathologic diagnosis is painful for the patient, slow, and subject to the availability of histopathologists.16 Frozen section with immunofluorescence may be a faster method but has not made the transition to clinical use.17 Ultrasonography has shown promise as a less painful modality that may help predict which partial-thickness burns may not heal.18 Laser Doppler imaging has generated much interest for measuring skin perfusion and is used to predict burn depth and potential for healing.19 Serial laser Doppler examinations have been reported to result in high specificity and positive predictive value when attempting to predict the healing of partial-thickness burns.20 Other technologies have been useful in laboratory investigations of burn depth, but methods using techniques such as indocyanine green angiography21 and optical coherence tomography22 are not practical for routine clinical use. It is important to remember that burn wound depth is dynamic, and may convert from superficial partial-thickness to deep partial thickness as a result of wound infection and even under-resuscitation, according to animal studies.23 Serial examinations of burns by an experienced surgeon are therefore mandatory throughout a patient’s hospital course.
BURN SIZE
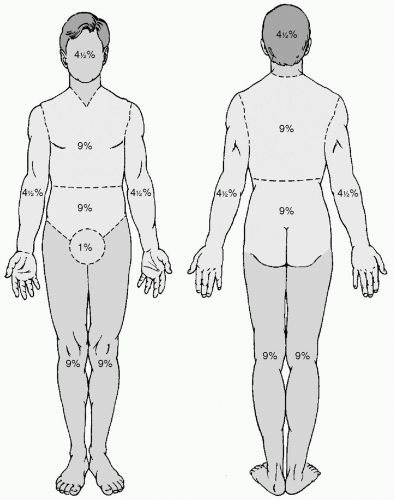
It is essential to make an accurate estimation of burn size to help determine resuscitation strategies and to give a preliminary indication of prognosis. Most formulas for resuscitation rely on the percent of body surface area burned (%TBSA). The “rule of nines” may give a quick estimation of burn size in the absence of other measuring tools (see Fig. 3). For adult patients, the anterior and posterior trunk each make up 18% of the TBSA, each lower extremity 18%, each upper extremity 9%, and the head 9%. The head in children may account for more of the total surface area. The patient’s palmar surface of the hand usually approximates 1% of the TBSA, and may be used to estimate total burn size. A card of standardized size with corresponding nomograms for modified body surface area has been reported as useful for rapidly and accurately assessing burn size.24 The Lund and Browder chart is a commonly used topographic diagram that is easy to use and accurate for estimating burn size.25
PROGNOSTIC INDICATORS
The classic equation for predicting mortality in burn patients in the past was simply that mortality equals patient age in years plus the %TBSA.26 Continuing advancements in burn care have markedly decreased mortality overall, resulting in overestimation of mortality by this formula. Clearly, age, depth of burn injury, and %TBSA, along with inhalation injury, are still major contributors to mortality. One review of 1,665 burn patients led to a mortality formula with three risk factors: age older than 60 years, more than 40% TBSA burned, and presence of inhalation injury. This accurately stratifies patients into four groups based on 0, 1, 2, or 3 risk factors.27 Mathematically adjusted functions based primarily on age also correlate with overall mortality in some series.28 Attempts have been made to apply other existing scoring systems for critically ill patients to the burn population, but correlation with mortality is poor. Adding a burn size factor to existing scoring systems may improve their predictive value.29 Female patients,
especially in the 30- to 59-year-old age-group, may have a higher mortality than their male counterparts in the same age-group.30
especially in the 30- to 59-year-old age-group, may have a higher mortality than their male counterparts in the same age-group.30
RECOMMENDATIONS FOR BURN CENTER TRANSFER
In an attempt to optimize care for burn patients, the ABA and the ACS have encouraged the transfer of patients with severe burns to a verified burn center. The established guidelines for transfer include partial-thickness burns of more than 10% TBSA, and/or third-degree burns in any age-group. Patients with burns involving the face, hands, feet, genitalia, perineum, or joints should be transferred, as well as patients with electrical or chemical burns or the presence of inhalation injury. Other patients who should be referred to a burn center include those with complicated preexisting medical disorders or those who will require specialized care for social, emotional, or rehabilitative needs. This includes children in hospitals not equipped for pediatric care. Patients with associated trauma, where traumatic injuries are the primary immediate threat, must be evaluated and stabilized at an appropriate trauma center before transfer to a burn center.31
INITIAL MANAGEMENT OF BURN INJURIES
There are several immediate measures that must be undertaken in the care of burn patients, the most important of which is attention to the airway. The larynx typically protects the subglottic airway from direct burns, but the supraglottic airway may be exposed to heat. Swelling in this area can be rapid and cause lethal airway obstruction. Signs of inhalation injury may include facial burns, singeing of the eyebrows and nasal hair, or carbon deposits in the oropharynx or sputum. A history of explosion or confinement in a closed space during a fire may also be a harbinger of inhalation injury.32 Known aspiration of hot liquid can precipitate a condition similar to epiglottitis and will require immediate intubation.33 A carboxyhemoglobin level of more than 10% should also warrant closer investigation of the airway. The presence of these markers, or any other clinical suspicion of inhalation injury, should prompt elective endotracheal intubation. A patient being transferred to a burn center should be intubated before transfer.
A crucial step in the immediate care of burn patients is to stop the burning process. All clothing should be removed because many synthetic fabrics can melt into a residue that may cause ongoing burning of the patient. Dry chemical powders should be carefully brushed from the wound, and all chemical burns should be irrigated with large quantities of water for at least 20 to 30 minutes.33 Expeditious placement of intravenous access is mandatory. Two large-caliber peripheral intravenous lines should be placed through nonburned skin if possible, although burned skin should not deter appropriate intravenous placement. If peripheral access is difficult, central venous catheters may be necessary. Intraosseous lines should be considered in both pediatric and adult patients34 with difficult venous access. Infusion of lactated Ringers solution should be started immediately. Discussion of resuscitation strategies will be covered later in this chapter, but regardless of formula used, unexpected hemodynamic lability during the initial evaluation may be an indication of other injuries and should prompt further investigation.
THE SECONDARY SURVEY AND ESCHAROTOMY
The secondary survey of the burned patient should include a thorough history and physical examination, with special attention paid to the burn size and depth. Evaluation of tissue perfusion is important, especially in circumferential extremity burns, in which performance of escharotomies may be necessary. Pain, numbness, delayed capillary refill, or loss of peripheral pulses may signal the need for escharotomy in the extremities. Circumferential abdominal eschar may lead to an abdominal compartment signaled by decreased urine output, hypotension, and increased airway pressures. Bedside escharotomies using simple electrocautery result in a visible release of underlying subcutaneous tissue and separation of the edges of the escharotomy line. Extra caution must be maintained to avoid injury in the areas of the upper arm (brachial artery), elbow (ulnar nerve), and knee (peroneal nerve). Thoracic escharotomies are done overlying the midline sternum and can be extended bilaterally in the subcostal areas. Lateral abdominal incisions release the abdomen and are easily extended to the lateral lower extremities if necessary. Extremity escharotomies are performed longitudinally on the medial and lateral aspects. Hand incisions extend to the thenar and hypothenar eminences, with digit escharotomies placed on the dorsolateral aspects of the fingers. Incomplete response of signs and symptoms after sufficiently generous escharotomies may signal the need for fasciotomies, which are typically performed in an operating room setting.35
Baseline laboratory studies should include a complete blood count (CBC), type and screen, carboxyhemoglobin, blood glucose, electrolytes, and pregnancy test in females of appropriate age. A chest x-ray may be indicated after endotracheal intubation or central line placement.33 A nasogastric tube should be considered, and an indwelling urinary catheter is mandatory to monitor urine output as an endpoint of resuscitation. It is occasionally necessary to incise a severely burned foreskin to permit access to the urethral meatus.36 Short-acting intravenous sedatives and narcotics should be used liberally for patient comfort. Initial coverage of the burns with clean linen helps keep
the patient warm and relieves pain from air currents, and unroofing of blisters or debridement of the wound should be postponed until arrival at the location of definitive care. Prophylactic systemic antibiotics are not indicated in the treatment of acute burns.33
the patient warm and relieves pain from air currents, and unroofing of blisters or debridement of the wound should be postponed until arrival at the location of definitive care. Prophylactic systemic antibiotics are not indicated in the treatment of acute burns.33
AIRWAY, BREATHING, AND VENTILATORY MANAGEMENT
Most patients with severe burn injury are intubated early for airway management and respiratory support. Pulmonary insufficiency and failure in severely burned patients are multifactorial.37 The etiology can be differentiated into direct pulmonary and upper airway inhalation injury and indirect or secondary acute lung injury due to activation of the systemic inflammatory response.37 Inhalation of hot air may cause upper airway, above vocal cords, burn that is particularly dangerous because swelling in this area can be rapid and cause lethal airway obstruction. In addition, inhalation of carbon particles, products of incomplete combustion, toxic gases, and organic acids can cause upper airway, lower airway, and alveolar injury. CO intoxication is a lethal complication of inhalation injury, which is covered at the end of this section. As already mentioned, any history of closed space fire should raise suspicion for inhalation injury. This is particularly important if there is history of prolonged exposure and confinement, such as being unconscious in a closed space fire. Early intubation before development of airway or pulmonary dysfunction is essential.
There is also secondary delayed pulmonary injury due to the systemic inflammatory response syndrome, sepsis, and pneumonia. In addition, ventilator-associated lung injury has been described as an important iatrogenic factor contributing to the secondary accentuation of pulmonary injury.38 The reduced pulmonary compliance and chest wall rigidity of burn patients can lead to aggressive ventilator management and high airway pressures, exacerbating acute lung injury. Institution of low-tidal volume ventilation, allowing permissive hypercapnia was shown to reduce the development of ventilator-associated lung injury and significantly improve outcomes.38 Additionally, use of alternate ventilation strategies such as high-frequency oscillatory and percussive ventilations may be beneficial in selected burn patients.39 Although there is limited comparative data regarding these two strategies, it appears that high-frequency percussive ventilation is especially useful in inhalation injuries.40
Clinical suspicion of inhalation injury can be quickly confirmed by fiber optic bronchoscopy. Significant findings include erythema, carbonaceous deposits, edema, bronchorrhea, and friability, which infrequently progresses to frank hemorrhage. Mucosal sloughing can lead to endoluminal obliteration.41 Adjunctive radiology is generally not helpful, although thoracic computed tomography (CT), 99Technetium (99Tc) scanning, and xenon scanning have been investigated for use in inhalation injury.42
Treatment of inhalation injury is still restricted primarily to supportive care. Inhaled β-agonists may ameliorate bronchospasm, and investigation continues regarding the use of other nebulized agents such as acetylcysteine and aerosolized heparin. These additional therapies have been shown to transiently improve pulmonary variables but have not impacted mortality.25 Steroids have no benefit after inhalation injury, and may actually worsen outcomes.43 Recombinant human antithrombin44 and aerosolized tissue plasminogen activator45 are effective in animal models of inhalation injury, but have not yet seen widespread clinical use. For patients with inhalation injuries that are refractory to treatment with conventional therapies, intrabronchial surfactant46 or inhaled nitric oxide47 may be useful in salvage situations.
An important and potentially lethal complication of inhalation injury is CO poisoning. It should be suspected in patients with inappropriate neurologic symptoms, and may be manifested by a “cherry red” appearance of the patient’s skin. Pulse oximetry is inaccurate in patients with CO poisoning, so carboxyhemoglobin levels must be obtained by arterial blood gas. A carboxyhemoglobin level of 5% or less is considered normal, and levels above 10% may be associated with clinical symptoms such as headache, confusion, and disorientation. Prolonged exposure or high levels of carboxyhemoglobin may be associated with significant complications and death. Therapy with 100% oxygen is the gold standard for elimination of CO. The half-life of CO is approximately 250 minutes in room air but drops to 40 to 60 minutes with administration of 100% oxygen.48 Hyperbaric oxygen therapy has been used in an attempt to improve neurologic outcomes in severe inhalation injury,49 but “diving” of critically ill patients often presents logistic difficulties that have limited its use in burn patients.
RESUSCITATION
Burn trauma leads to a combination of hypovolemic and distributive shock on the basis of generalized microvascular injury and interstitial third-spacing through collagen and matrix degeneration.37 Burn injury is marked by a dynamic and ongoing fluid shift that has led to the development of fluid resuscitation formulas based on percentage of TBSA burn and weight. Fluid resuscitation based on the Parkland burn formula is extensively used in burn centers and has helped minimize the occurrence of burn shock.50 Most surgeons agree that the estimated need of a burn patient in the first 24 hours is 2 to 4 mL/kg/% body surface burn, half of which is given in the first 8 hours and the other half is given in subsequent 16 hours. Therefore, a 100 kg patient with 30% body surface area burn will require 6 to 12 L of fluid, commonly warm Ringers lactate, in the first 24 hours. However, a
recent survey of 28 burn centers found that in 58% of patients actual fluid resuscitation exceeded the 4 mL per kg recommended by Baxter.51 Over-resuscitation has been shown to correlate directly with rising intra-abdominal pressure and the development of abdominal compartment syndrome.52 Apart from the well-described extremity compartment syndrome, orbital compartment syndrome requiring canthotomy in patients receiving supranormal resuscitation was recently described.53 The current high-volume fluid regimens have shifted postburn resuscitation complications from renal failure to pulmonary edema with increased requirement for ventilatory support, the need for fasciotomies in unburned limbs, and the occurrence of the abdominal compartment syndrome.54
recent survey of 28 burn centers found that in 58% of patients actual fluid resuscitation exceeded the 4 mL per kg recommended by Baxter.51 Over-resuscitation has been shown to correlate directly with rising intra-abdominal pressure and the development of abdominal compartment syndrome.52 Apart from the well-described extremity compartment syndrome, orbital compartment syndrome requiring canthotomy in patients receiving supranormal resuscitation was recently described.53 The current high-volume fluid regimens have shifted postburn resuscitation complications from renal failure to pulmonary edema with increased requirement for ventilatory support, the need for fasciotomies in unburned limbs, and the occurrence of the abdominal compartment syndrome.54
Warm crystalloid solutions such as lactated Ringers solution and normal saline are still first line fluid replacements in burn resuscitation.37 Owing to reduced intravascular fluid retention, large volumes have to be infused, which accentuate tissue edema, and the development of tissue edema can lead to worsening outcomes.55 Hypertonic saline (HTS) resuscitation (7.5% NaCl) has been promoted for its efficient intravascular volume resuscitation, rapid restoration of blood pressure and cardiac output with improved cerebral perfusion, and potential for expanding circulating volume by reabsorption of fluid from the interstitial space.56 More recently, HTS has been studied as a fluid with significant modulation of systemic inflammatory response secondary to reperfusion injury, which may be beneficial in patients with shock.57,58 In animal models of trauma and burn, use of HTS resuscitation has been associated with decreased edema and improved organ perfusion and outcomes.59,60,61 Data on the effectiveness of HTS to prevent organ damage in the clinical setting are inconsistent.62 Some of the studies in burn patients have demonstrated that HTS may decrease the fluid load, tissue edema, and complications such as abdominal compartment syndrome.63,64,65 However, others have noted no benefit in fluid requirement and potentially increase the risk of renal failure.66 It appears that the benefit of HTS is seen in early resuscitation,67 and some researchers have recommended stopping HTS infusion when serum sodium concentration exceeds 160 mEq per L.54,68 Currently, HTS is not routinely used in burn patients, and further research is required to better define potential benefits, the timing, and the optimal volume.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree