CHAPTER 12
Botulinum Toxin in the Treatment of Upper Limb Spasticity
Allison Brashear
The use of botulinum toxin (BoNT) to treat spasticity of the upper extremity has dramatically improved the care of patients with increased tone as part of the upper motor neuron syndrome (UMNS). Before the introduction of BoNT, chemodenervation was only performed by relatively few physicians with injections of phenol and alcohol. The limited access to those performing these injections coupled with concerns about pain and long-term sequelae left many patients without adequate treatment of their UMNS. The introduction of BoNT for the treatment of increased tone has greatly increased the ability of physicians to manage spasticity of the UMNS, focusing on symptoms that interfere with activities of daily living and the ability of the caregiver to care for the patient. Today, the use of BoNT for the treatment of tone in patients with UMNS is one of the most effective and well-tolerated tools physicians use to treat this disabling medical problem (1).
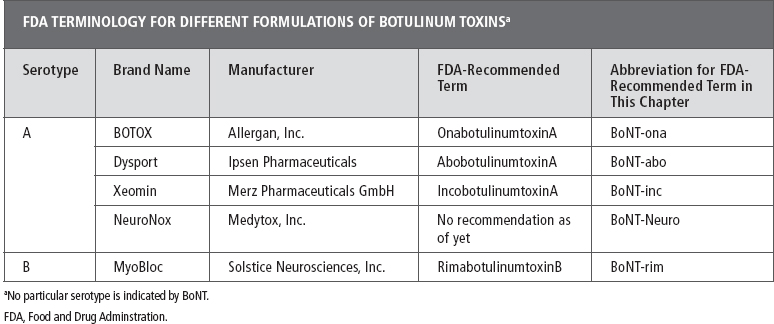
The first introduction of BoNT (formulated as onabotulinumtoxinA; see Table 12.1) to the U.S. market came in 1989 with the approval of BoNT-ona in the United States for injection in the face and eye muscles. Dr Scott and his colleagues developed BoNT-ona as a drug for a temporary treatment of strabismus in children. Dr Scott theorized that small amounts of the potent toxin could temporarily cause relaxation of the injected eye muscle (2–4). Later, the adaptation of BoNT to treat overactive muscles in the neck, jaw, and limbs was spearheaded by physicians seeking to treat focal medically refractory increases in muscle tone in a variety of movement disorders (5–7). The ability to select specific muscles, titrate doses for selective relaxation, and avoid systemic side effects allows physicians to treat overactive muscles in many diseases in which there had been no successful treatment before the introduction of BoNT (8). Improvement of function is the cornerstone of treatment with BoNT. Function may be active such as grip or passive such as hand hygiene by a caregiver. Generally, these outcomes are individualized per the Goal Attainment Scale (9). Treatment of upper extremity spasticity with BoNT enables physicians to focus on specific activities and functions important to the patient and/or caregiver (10). The definition of function varies from patient to patient, and as a result, it is important for clinicians to individualize their treatment approach for each patient. Although all patients with upper extremity spasticity have increased tone, the pattern of presentation may vary by etiology. Increased tone is part of the larger UMNS that may also include weakness and incoordination (11). Likewise, the ability of the patient to participate in adjunct therapy after BoNT treatment may also vary by etiology. For example, central nervous system injury from traumatic brain injury or cerebral palsy may preclude some form of occupational therapy due to cognitive impairment, whereas those with poststroke spasticity may have spatial, language, or speech issues interfering with therapy. Some investigators are examining when patients should be treated, including more immediate after stroke, combining injections with stimulation or constraint induced therapy (12,13).
The heterogeneous presentations of upper extremity spasticity have led some investigators to focus on the treatment of spasticity by etiology. As a result, many trials of upper extremity spasticity are limited to common diseases, such as poststroke spasticity. To study a more homogeneous group, many studies have limited enrollment to poststroke spasticity, and further studies may often limit enrollment to those patients requiring sufficient communication skills to complete scales or perform tasks. In clinical practice, the dosing, technique, and benefits noted in the poststroke spasticity trials have been extrapolated to those with traumatic brain injury and cerebral palsy, but trials including adults with spasticity due to traumatic brain injury, adult cerebral palsy, and multiple sclerosis have been small. Therefore, the focus of this chapter is on poststroke spasticity, but the reader is urged to keep the other causes of the UMNS in mind (14–16).
TABLE 12.1
MECHANISM OF ACTION OF BONTS
BoNTs are protein neurotoxins produced by several different strains of the Clostridium botulinum bacterial species with varying serotype (designated types A through G [17]). Only serotypes A and B have been developed for routine use in health care (18). Within a serotype, the production varies by manufacturers, and, therefore, no generic equivalent is available for these toxins. In addition, within serotype A, there are different formulations with different standard doses, and the use of one formulation cannot be easily substituted for another (19). It is important to note that of the commercially available toxin products, the biological activity units are unique to each BoNT preparation and cannot be compared or converted into another. For example, if a patient is “switching” from onabotulinumtoxinA to rimabotulinumtoxinA, the physician should assume that they are starting “new” on the dose titration.
The mechanism of action of all BoNT serotypes is to inhibit the vesicle-dependent release of acetylcholine and other neurotransmitters from the presynaptic nerve terminal. The binding of the vesicle-containing acetylcholine and other transmitters requires a complex set of proteins called “SNARE” proteins (soluble N-ethylmaleimide-sensitive factor attachment protein receptor), which include synaptobrevin (inhibited by serotypes B, D, F, and G); SNAP-25 (inhibited by BoNT-A, -C, and -E); and syntaxin (inhibited by serotype C) (20). The light chain of each serotype acts at a distinct site for one or more of the proteins required for vesicle release. Even when the same protein is affected, as in serotypes A, C, and E, the different serotypes affect the protein at a different site.
In a normally functioning presynaptic nerve terminal, transmitters from the vesicle are released into the synaptic cleft and then bound by receptors on the muscle cell. All of the serotypes of BoNT interfere with this process. The effect of BoNT injection is inhibition of acetylcholine release into the presynaptic cleft. As a result, the postsynaptic membrane of the muscle perceives no input from the nerve terminal and thus becomes “chemically denervated” (20).
CONCERNS ABOUT DOSING
The use of BoNT for the treatment of spasticity involves large doses of toxin and always has potential for systemic spread. In 2009, the U.S. Food and Drug Administration (FDA) mandated a new label warning and risk mitigation strategy for all BoNT sold in the United Sates (21). A release from the FDA noted that it instigated the labeling change due to reports of systemic spread in some patients and the potential for serious risks associated with the lack of interchangeability among the three licensed BoNT products (BOTOX®, Dysport®, and MyoBloc®/NeuroBloc®). According to the FDA website, reports of “spread of toxin has been reported to other areas of the body causing symptoms similar to those of botulism, including unexpected loss of strength or muscle weakness, hoarseness or trouble talking, trouble saying words clearly, loss of bladder control, trouble breathing, trouble swallowing, double vision, blurred vision and drooping eyelids. These symptoms have mostly been reported in children with cerebral palsy being treated with the products for muscle spasticity, an unapproved use of the drugs. Symptoms have also been reported in adults treated both for approved and unapproved uses” (21). The FDA recommended that health care professionals who use BoNTs should do the following: “understand that dosage strength (potency) expressed in ‘Units’ is different among the botulinum toxin products; clinical doses expressed in units are not interchangeable from one product to another; be alert to and educate patients and caregivers about the potential for effects following administration of botulinum toxins such as unexpected loss of strength or muscle weakness, hoarseness or trouble talking, trouble saying words clearly, loss of bladder control, trouble breathing, trouble swallowing, double vision, blurred vision, and drooping eyelids; understand that these effects have been reported as early as several hours and as late as several weeks after treatment; and advise patients to seek immediate medical attention if they develop any of these symptoms” (21).
WHY DOES BoNT WORK FOR SPASTICITY?
Currently, there are four main BoNT products available worldwide for clinical use: BOTOX (Allergan, Inc., Irvine, CA); Dysport (Ipsen Pharmaceuticals, Slough, UK); MyoBloc/NeuroBloc® (Solstice Neurosciences, Inc., South San Francisco, CA/Solstice Neurosciences Ltd., Dublin, Ireland); and Xeomin® (Merz Pharmaceuticals GmbH, Frankfurt, Germany). Of the four products, three are BoNT serotype A (BoNT-A) products (BOTOX, Dysport, and Xeomin), whereas MyoBloc is a BoNT serotype B (BoNT-rim) product. More recently, NeuroNox (BoNT-Neuro) is a new form of serotype A BoNT. NeuroNox is manufactured by Medytox Inc. (Ochang-eup, Cheongwon-gu, Cheongju-si, Chungcheongbuk-do, Republic of Korea) and is also sold as Meditoxin in Korea. In 2009, the FDA suggested names to identify the different formulations of BoNT. BoNT type A (formulated as BOTOX) is to be referred to as onabotulinumtoxinA. BoNT type A formulated as Dysport is to be referred to as abobotulinumtoxinA, the product that is formulated as Xeomin, is to be referred to as incobotulinumtoxinA. A different serotype, BoNT type B formulated as MyoBloc is to be referred to as rimabotulinumtoxinB (Table 12.1). Although no generic forms of BoNT exist, these terms are designed to steer away from brand name terminology in literature and continued medical education (CME)-accredited events. The adoption of this terminology is usually used in CME-approved courses. There is no FDA terminology as of this writing for the new Korean toxin NeuroNox.
A majority of the work in the literature in upper extremity spasticity is with either BoNT-ona or BoNT-abo—both are type A toxins. BoNT-inc is the newest type A toxin to appear on the market, and most of the work to date has been limited to studies in blepharospasm and cervical dystonia. Small trials have explored the use of BoNT-rim in spasticity, but large trials with serotype B in spasticity have not been published (22,23).
Early Studies of BoNT-ona in Poststroke Spasticity
In a very early study in 1996, Simpson et al (24) published a small double-blind, placebo-controlled, multicenter trial of BoNT-ona in 39 patients with poststroke spasticity. In this dose-finding study, the authors sought to determine the minimally effective dose to treat spasticity at the wrist and elbow in patients with spasticity naive to any BoNT treatment. All patients were at least 9 months poststroke and received treatment of the elbow and wrist flexors only. The finger flexors were not treated. Dosing groups included a total dose of 75, 150, or 300 U of BoNT-ona compared with placebo. The largest dosage group (300 U BoNT-ona) resulted in a statistically and clinically significant mean decrease in wrist flexor tone of 1.2 points (P = .028), 1.1 points (P = .044), and 1.2 points (P = .026) and elbow flexor tone of 1.2 points (P = .024), 1.2 points (P = .028), and 1.1 points (P = .199) at weeks 2, 4, and 6 postinjection. The lower two dosage groups of BoNT-ona showed some improvement in tone but did not reach statistical significance. No appreciable changes were noted in the goniometry measurements or functional measures. Adverse effects were limited and well tolerated.
In a multicenter randomized, placebo-controlled, dose-ranging study of three doses of BoNT-ona across all three joints, Childers et al (25) studied treatment of elbow, wrist, and finger flexors with three doses (90, 180, and 360 U) compared to placebo. The primary outcome was the Modified Ashworth (0–4 with half points allowed), a patient’s, and a physician’s global rating of change, and a frequency of pain rating scale (0–5), and quality-of-life scales. Patients received up to two treatments of either placebo or a total dose of 90, 180, or 360 U. Patients were followed up for a total of 24 weeks. The Modified Ashworth (0–4 with half point allowed), pain, 36-item short form, and functional independence measures were assessed at 6, 9, 12, and 24 weeks posttreatment. The muscle injection included biceps (50–200 U), flexor digitorum sublimis (7.5–30 U), flexor digitorum profundus (7.5–30 U), flexor carpi radialis (15–60 U), and flexor carpi ulnaris (15–60 U) and were all given with electromyographic guidance. Of the 19 centers involved, a total of 91 subjects enrolled with 77 patients completing the study (25).
As noted in the earlier Simpson study, the highest total dose of BoNT-ona (360 U) produced the greatest improvement in muscle tone. Those treated with the 180 and 90 U dosages demonstrated less reduction in tone. There were no changes in the 36-item short form, functional independence measure, or life scores. The results of the Simpson and Childers study demonstrated the need for sufficient dosing at multiple joints in the spastic limb. Although a decrease in tone was documented by the Ashworth (see Table 12.2) and Modified Ashworth, neither study demonstrated a functional change.
In 2002, the largest double-blind, placebo-controlled study of toxin-naive patients with spasticity of the wrist and fingers after stroke demonstrated sustained improvement with onabotulinumtoxinA more than 12 weeks in muscle tone and disability. In addition, Brashear et al (26) used a novel scale specifically developed for upper extremity BoNT studies, the Disability Assessment Scale (DAS; see Table 12.3). The DAS was administered with the patient and/or caregiver and physician together rating the impairment of hand hygiene, pain in the hand, look of the hand (cosmesis), or use of the hand in dressing on a simple 0-to-3 scale. The patients selected one (dressing, hygiene, pain, or cosmesis) as the most important disability to improve with the treatment. The agreed upon subscale of the DAS was followed as a secondary outcome measure in the study. As in other large trials with BoNT, the primary outcome measure remained muscle tone as measured by the Ashworth. Since 2002, the DAS has been incorporated in more trials but the Ashworth or the Modified Ashworth remains the primary outcome in most studies (27,28).
TABLE 12.2
ASHWORTH SCALE |
0 = No increase in tone (none) |
1 = Slight increase in tone, giving a catch when the limb is moved in flexion or extension (mild) |
2 = More marked increase in tone but limb is easily flexed (moderate) |
3 = Considerable increase in muscle tone (passive movement difficult, severe) |
4 = Limb rigid in flexion or extension (very severe) |
Source: From Ref. (26). Brashear A, Zafonte R, Corcoran M, et al. Inter- and intrarater reliability of the Ashworth Scale and the Disability Assessment Scale in patients with upperlimb poststroke spasticity. Arch Phys Med Rehabil. 2002;83(10):1349–1354.
At the end of 12 weeks, subjects treated with BoNT-ona had greater improvement in wrist and finger flexor tone at each visit than did the placebo group. The maximal difference between the two groups in the mean change from baseline occurred at week 4 (wrist: –1.78 active group, –0.42 placebo group [P < .001]; finger: –1.59 active, –0.27 placebo group [P < .001]). Subjects who received BoNT-ona demonstrated a decrease in muscle tone compared to placebo at all-time points in the study (P < .001 [29]).
The preselected goal of treatment (one subscale of the DAS) demonstrated greater improvement in the treated group than those treated with placebo. At 6 weeks after injection, 40 patients of the active group (62%), as compared to 17 patients in the placebo group (27%), had improvement in their chosen subscale of the DAS. In addition, the treated group demonstrated similar improvement in the DAS subscale at weeks 4 (P < .001), 6 (P < .001), 8 (P = .03), and 12 (P = .02). In addition, there was an improvement in all areas measured by the DAS (pain, limb position, hygiene, and dressing) in those treated with drug. Six weeks after injection, 53 (83%) of 64 patients had at least 1-point improvement on the DAS in one or more areas compared with 33 (53%) of 62 patients who received placebo (P = .007 [13,29]).
TABLE 12.3
DISABILITY ASSESSMENT SCALE |
Hygiene: Assess the extent of maceration, ulceration, and/or palmar infection; palm and hand cleanliness; ease of cleanliness; ease of nail trimming; and the degree of interference caused by hygiene-related disability in the patient’s daily life. |
Dressing: Assess the difficulty or ease with which the patient could put on clothing and the degree of interference caused by dressing-related disability in the patient’s daily life. |
Limb position: Assess the amount of abnormal position of the upper limb. |
Pain assess Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|



