Fig. 8.1
Diabetes and bone-generated hormone interaction via osteoblast/osteocyte impairment. Several factors associated with diabetes impair osteoblast/osteocyte differentiation and function where FGF23 and osteocalcin were generated. AGEs advanced glycation end products, MSC mesenchymal stem cell
8.1.3 Regulation of Phosphorus Metabolism
Serum phosphorus levels are mainly regulated by phosphorus absorption from the digestive tract and phosphorus reabsorption in the renal tubule (Fig. 8.2). Most of the phosphorus filtered in the glomerulus is reabsorbed in the proximal renal tubule. Type 2a and 2c sodium–phosphate cotransporters are responsible for the physiological reabsorption of phosphorus in the proximal renal tubule. FGF23 acts on the Klotho–FGF receptor (FGFR) complex in the renal tubule and suppresses phosphorus reabsorption by decreasing the expression of type 2a and 2c sodium–phosphate cotransporters [7]. In the kidney of streptozotocin-induced diabetic rats, decrease of Klotho and FGFR expression by high glucose has been documented [8]. FGF23 also decreases the level of activated vitamin D3 [1,25(OH)2D3], which accelerates phosphorous absorption from the digestive tract, by decreasing the expression of Cyp27b1 (1α-hydroxylase), an enzyme producing 1,25(OH)2D3, and by facilitating the expression of Cyp24 (24-hydroxylase), which transforms 1,25(OH)2D3 into a metabolite with lower activity [9]. As shown above, FGF23 decreases serum phosphorus levels by suppressing phosphorus reabsorption in the renal tubule as well as phosphorus absorption from the digestive tract by decreasing the serum 1,25(OH)2D3 level.
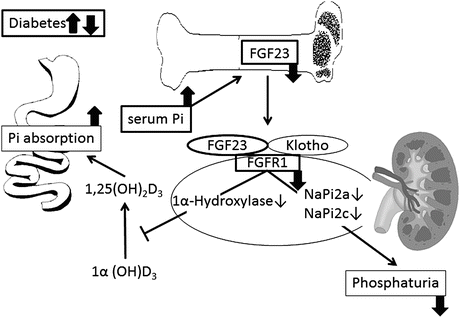

Fig. 8.2
Possible influence of diabetes on the regulation and action of FGF23. Increased serum Pi causes FGF23 to be released from skeletal osteoblasts/osteocytes. The major known effects of FGF23 are inhibition of Na–Pi cotransport in the kidney and the resultant phosphaturia and inhibition of 1α(OH)D3 hydroxylase, which reduces levels of activated vitamin D3 [1,25(OH)2D3]. Reduced 1,25(OH)2D3 levels decrease gastrointestinal Pi absorption. In diabetes, impaired production of FGF23 at osteoblasts/osteocytes and downregulation of FGF23-specific receptor that is composed of Klotho and FGF receptor 1 (FGFR1) may lead to decrement in phosphaturia and increment in Pi absorption
We previously reported a decrease in FGF23 responsiveness to phosphorus loading in patients with diabetes (Fig. 8.3) [10]. Phosphorus (1 g) was orally administered to patients with type 2 diabetes (n = 10) and nondiabetic patients (n = 10), and then, the short-term effect was examined. Patients in both groups were confirmed to have no renal dysfunction. The serum FGF23 level was significantly increased in the nondiabetic group 2 and 4 h after the administration of phosphorus, whereas no increase was observed in the diabetic group. The serum iPTH level also increased significantly in the nondiabetic group 4 h after the load, whereas no increase was seen in the diabetic group. Serum phosphorus levels were significantly increased in both groups 2 h after phosphorus administration. Although the serum phosphorus level continued to increase in the diabetic group up to 4 h after the administration, it was suppressed in the nondiabetic group. Next, the long-term effect of phosphorus loading was examined by administering phosphorus (2 g per day) orally on two consecutive days. Significant increases in both serum FGF23 and iPTH levels were observed only in the nondiabetic group 3 days after the administration. When phosphorus was orally administered for 7 days to patients with chronic renal failure, the serum FGF23 level continued to increase from the basal value in the nondiabetic group during the investigation, whereas no such change was seen in the diabetic group [11].
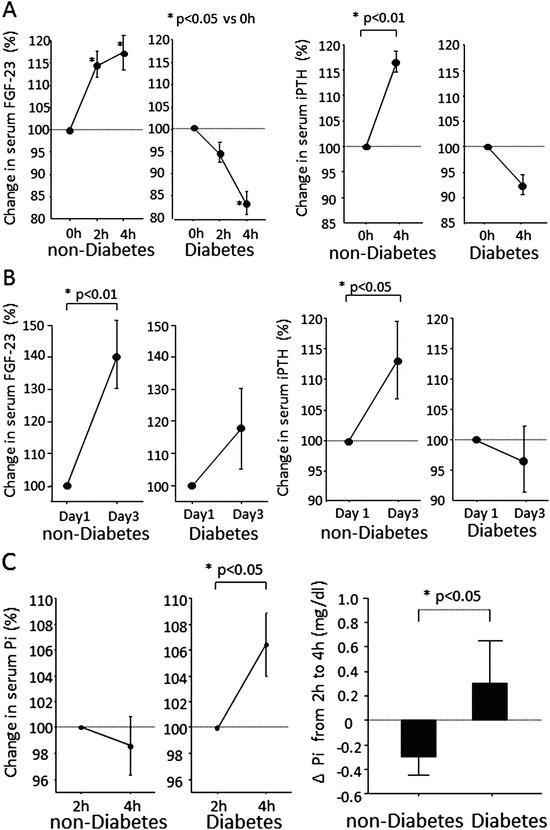

Fig. 8.3
Impaired responses of serum FGF23 and iPTH to oral Pi stimulation test. (a) Time courses of serum FGF23 and iPTH from 0 to 4 h after intake of Pi on day 1. In nondiabetic patients, serum FGF23 elevated significantly (P = 0.046 by ANOVA) and showed a significant increase at 2 h after Pi stimulation (P < 0.05 by Fisher test). In contrast, serum FGF23 was significantly reduced in diabetic patients (P = 0.018 by ANOVA) and showed a significant decrease at 4 h (P < 0.05 by Fisher test). During oral Pi stimulation, serum iPTH significantly increased from 0 to 4 h in nondiabetic patients (P = 0.007) but not in diabetic patients (P = 0.072). (b) Comparison of serum FGF23 and iPTH levels on day 1 (0 h) and day 3 (0 h). An oral Pi load for 2 days significantly increased serum FGF23 and iPTH in nondiabetic patients (P = 0.009, P = 0.048) but not in diabetic patients (P = 0.241, P = 0.507). (c) Comparison of serum Pi changes in nondiabetic and diabetic patients. Serum Pi significantly increased between 2 and 4 h in diabetic patients (P = 0.020), whereas there was no significant change in nondiabetic patients (P = 0.574). The serum Pi changes from 2 to 4 h differed significantly between the two groups (P = 0.027)
8.1.4 Association with Arteriosclerosis
Because an increase in the serum phosphorus level is a risk factor for cardiovascular calcification and reduced life expectancy, the serum FGF23 level, which decreases the serum phosphorus level, helps predict the onset of cardiovascular calcification and reduced life expectancy. Chronic renal failure is one of the pathological conditions in which the serum phosphorus level is increased. When renal function decreases, this phosphaturic effect is hindered, and phosphorus accumulates in the body. In chronic renal failure, FGF23 increases when the estimated glomerular filtration rate reaches around 60 mL/min, which is before PTH [12], and this protects against an increase in the serum phosphorus level. For this reason, the FGF23 increase is a predictive factor for the progression of chronic renal failure, which is independent of the amount of albumin excreted into the urine [13]. When renal failure reaches the advanced stage, FGF23 levels are progressively increased to compensate for persistent phosphate retention, but this results in reduced renal production of activated vitamin D and decreased serum Ca and leads to secondary hyperparathyroidism (Fig. 8.4). As a result, Ca and phosphorus are recruited from bone to blood and increase the risk of cardiovascular calcification. Vessel calcification caused by this mechanism is called Monckeberg medial calcific sclerosis to distinguish it from the atherosclerotic plaques in the vascular intima. It is characterized by calcification within the vascular media. Increased areas of Monckeberg calcification are involved in the onset of cardiovascular events and the increase in the mortality rate [14]. The frequency of Monckeberg calcification in the peripheral artery (the artery in the hand) [15] and in the abdominal artery [16] reportedly increases in diabetic patients compared with nondiabetic patients. This suggests diabetes is a state where it is easy to accumulate phosphate and leads to secondary hyperparathyroidism. Based on this background, the increase in serum FGF23 can serve as a predictive factor for total death, in addition to cardiovascular events, in patients with chronic renal failure [17] or in patients on dialysis [18].
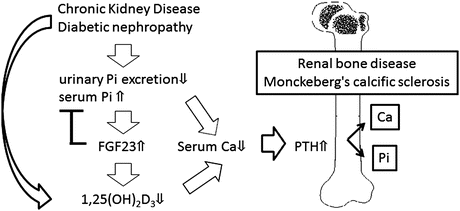

Fig. 8.4
The role of FGF23 in chronic kidney disease – mineral and bone disorder. In patients with chronic kidney disease, circulating FGF23 levels are progressively increased to compensate for persistent phosphate retention, but this results in reduced renal production of activated vitamin D3 [1,25(OH)2D3] and leads to secondary hyperparathyroidism. Ca and Pi are recruited from bone to blood and increase the risk of cardiovascular calcification known as Monckeberg calcific sclerosis
Some studies have suggested the direct involvement of FGF23 in the progression of vascular calcification, in addition to its indirect involvement via phosphorus metabolism. The FGF23 signal is transmitted through its binding to the Klotho–FGFR complex, which consists of membrane-bound Klotho and FGFR-1 and FGFR-3. Lim et al. reported that the Klotho–FGFR complex was expressed not only in the renal tubule but also in the arteries of healthy people [19]. When examined in arterial smooth muscle cells, the sensitivity of the Klotho–FGFR complex toward FGF23 decreased, and the calcification of vascular media was accelerated under the condition of high glucose and/or uremia. Even in clinical trials, the decrease in FGF23 was reported to be a risk factor, independent of the increase in the Ca/phosphorus product, of the calcification of peripheral arteries [20] and the arch aorta [21], and these reports support the direct involvement of FGF23 in suppressing vascular calcification.
8.2 Osteocalcin
8.2.1 Overview
Osteocalcin was identified as a bone matrix protein mainly secreted by osteoblasts. Osteocalcin is carboxylated by γ-carboxylase, and most of it is embedded within the bone as part of the bone matrix. In blood, osteocalcin exists in the following two forms: one with all three glutamic acid residues carboxylated and the other with less carboxylation of the residues. Undercarboxylated osteocalcin (ucOC) facilitates the synthesis and secretion of insulin in the pancreas and increases the insulin sensitivity of peripheral tissues. In addition, insulin signaling in osteoblasts activates osteocalcin by regulating the interaction between osteoblasts and osteoclasts. In this manner, bone tissue creates a positive feedback mechanism that acts on pancreatic β-cells and adipose tissues via hormones such as ucOC and insulin. Some reports on clinical studies in humans have also suggested that ucOC facilitates insulin secretion and enhances insulin sensitivity.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







