Biomaterials Overview
Kathleen A. Lewicki
Douglas W. Van Citters
Introduction
Given the omnipresence of the term “biomaterials” in our lexicon and in our day-to-day interactions with patients, it is remarkable to consider that the formal study of biomaterials is a relatively young field. However, while biomaterials departments, journals, and societies trace their official beginnings in the mid-1970s, the field actually finds its roots in orthopedics over 4,000 years ago. The first reports of natural adhesive bandages and quick-setting plaster can be found in the Edwin Smith Papyrus, and actual evidence of bandaging has been found in the tombs of the pharaohs.
Prior to aseptic surgical technique, however, use of biomaterials in internal musculoskeletal applications could often be a death sentence, with mortality rates approaching 50% (1). Thus, for thousands of years, the advancement of the field was limited to treatment of external wounds and the oral cavity. The great turning point in biomaterials’ progress, and in turn orthopedic biomaterials was seen with the proliferation of Lister’s sterile technique (1860s). Early reports of orthopedic biomaterials include Hansman’s use of nickel-plated steel for fracture fixation (1886), Gluck’s use of an ivory ball to replace the femoral head (1891), and Lane’s use of steel screws and plates for fracture fixation (1893) (2,3). The development of corrosion-resistant metals (early 1900s), however, provided surgeons with the ability to effectively place a material in the body, close the wound around the material, and leave it for long durations. The first corrosion-resistant metals were stainless steels, followed closely by cobalt-based alloys.
It is remarkable to consider that nearly 100 years later, surgeons are still using the same general classes of materials. Charnley low-friction arthroplasty first employed stainless steel and polytetrafluoroethylene (PTFE). Because of high wear rates and near-universal adverse tissue reactions to wear debris, the Charnley design quickly evolved to include a bearing surface of ultra-high molecular weight polyethylene (UHMWPE) in the early 1960s, and a femoral stem of the aerospace alloy cobalt-chrome-molybdenum (CoCrMo). Thus, the materials of the modern total hip arthroplasty were developed and in service prior to the advent of biomaterials’ regulation in the United States (4) and have only experienced evolution since then.
The following chapter explores the materials used in today’s total hip arthroplasty, focusing on the needs and specifications of the surgeon and patient. Although commercially available biomaterials in the hip can be broadly categorized into polymers, ceramics, and metals, they are perhaps best examined through their function as bearing surfaces, support systems, or fixation approaches. Each application area will be examined in detail with particular attention paid to bearing surfaces. Ongoing research and the future of biomaterials in the hip are also presented.
The Engineer’s Challenge
Mechanical design and material specification in the adult hip are some of the more difficult challenges faced by surgeons and biomedical engineers. The environment is notoriously aggressive, delivering mechanical, tribologic, and corrosive challenges to arthroplasty systems. Although the biomechanics and biologic environment of the hip are beyond the scope of this chapter, it is worth noting that arthroplasty systems must be designed to accommodate the following:
High loads transferred through small areas: During gait, an average adult might transfer two to three times their body weight through the lower extremity joints (5). Owing to the relatively small geometry of the acetabulum, these forces can translate to quite high contact stresses in a total joint bearing surface, and quite high bending stresses in the femoral stem and neck. Less frequent activities such as running, jumping, descending stairs, or stumbling can deliver forces approaching 10 times the body weight (6). As the activity level and the body mass index of the patient population increase, so shall the stresses delivered to the prosthesis. Implant materials must therefore be selected to account for their fracture toughness.
A corrosive environment: The sodium chloride concentration in blood of 0.9% can have a dramatic electrochemical effect on implanted devices, particularly because of the chloride ion in solution. Other reactive species are readily available, including peroxides and oxidized lipids. Although corrosion can directly impact the mechanical
properties of a material, corrosion by-products can be potentially harmful to the host. Implant materials must be relatively inert.
A high motion environment: Joint recipients are known to take over a million steps per year, though many recipients show higher activity levels; some taking over 3.2 million steps per year (7). Devices must therefore be exceptionally fatigue- and wear-resistant.
Numerous other factors must be considered, including cost, density, machinability, and ease of sterilization.
Supporting Structures in the Femur and Acetabulum
Although the functional goal of joint arthroplasty is to return a patient to activities of daily living and range of motion in the absence of pain, removal or replacement of solely the diseased or damaged portion of the joint is inadequate. Thus, support structures such as the femoral stem and the acetabular shell are employed to transfer load between the remaining, healthy bone stock and a new artificial bearing surface. Typically, these components are metallic.
Three classes of alloys are listed in the FDA-recognized ASTM Consensus Standards (ASTM F2068) for meeting the aforementioned specifications for use as stems or shells. They include stainless steels, cobalt-based alloys, and titanium-based alloys.
As previously mentioned, stainless steel was the first class of alloy introduced for orthopedic implants (8). Current ASTM standards recommend variants of type 316L, a low carbon alloy with chrome and nickel added (9). This austenitic steel has good corrosion resistance because of its low carbon content and molybdenum and chromium components. The resistance to oxidation coupled with relative ease of machining, forming, and hardening makes stainless steel a strong candidate for material choice. However, since some corrosion is inevitable, it has been recommended that stainless steel only be used for short-duration purposes (9). Few joint arthroplasty devices in the United States incorporate stainless steel for this reason, though stainless devices remain available in other countries.
Cobalt alloys found their beginnings in dentistry and are now one of the major materials used for hip prostheses. These alloys are exceptionally tough, though they are susceptible to work hardening and can easily become brittle during processing. As such, it is difficult to machine implants, which instead must be cast or wrought (10). However, the favorable strength, corrosion, and wear characteristics make alloys of CoCrMo a primary choice as an implant material.
The final alloy class used in orthopedics is titanium based. The most popular alloy in implants is Ti-6Al-4V, which has good corrosion resistance, high strength, and low density (9). Titanium alloys are multiphase metals and have a tendency to change physical properties when coated with a porous material (11). Nonetheless, titanium’s superior, mechanical, and chemical properties make it a widely used material in implant fabrication.
Cobalt-Chromium Alloys
CoCr alloys are produced in multiple compositions for implants. Variations in composition are typically tailored to accommodate the mechanism of forming the metal into its final shape and the desired properties of the final product. For instance, Co28Cr6Mo must be cast to retain its desirable properties (12) whereas Co35Ni20Cr10Mo can be wrought and cold-worked (13). Both alloys derive much of their strength from precipitation hardening (12), a phenomenon in which particles in the crystalline matrix serve to counter the effects of defects in the crystalline structure.
Cast CoCr has been formed through a lost-wax casting method since the material was first introduced in the 1930s (8). Although the exact methods and machines have changed over the past 70 years, the general technique remains unchanged. First, a mold of the part to be manufactured is made using brass. This mold is used to cast wax replicas of the final specimen, which are then coated with a ceramic shell. The wax is then melted out of the ceramic to leave a perfect one-piece mold for the final product. Liquid CoCr alloy is injected into the mold and cooled at a controlled rate to achieve the desired microstructure. The ceramic is sacrificed and the part is ready for final polishing.
CoCrMo has a coarse dendritic microstructure immediately after casting (14). The nondendritic areas are a face-centered cubic (FCC) cobalt matrix containing carbide and sigma phase (12). Metallurgists are careful to avoid a transition to the epsilon phase during treatment since its hexagonal close-packed (HCP) structure promotes more carbide growth (the literature identifies the carbide as M23C6 where M represents Co, Cr, or Mo) (13). Carbides (Fig. 12.1) often cited as a primary mechanism used to harden steels, make the CoCr alloy too brittle for use, and are therefore undesirable without appropriate processing. After forming, annealing or hot isostatic pressing can be used to modify the microstructure and improve the mechanical properties of the metal (12).
Mechanical properties for cobalt alloys have been specified by ASTM, but span a significant range. For instance, the ultimate strength for cobalt ranges from 655 MPa in the cast state to 1793 MPa in the wrought and cold-worked state (9). Yield strengths are accordingly varied from 450 to 1,585 MPa (9). Thus, a manufacturer has significant leeway in selecting a material and processing method to tailor the alloy to a specific application and design.
Cobalt oxidizes readily when placed in the human body. However, chromium oxide changes the overall electrochemical properties of the implant (15). As a result, once the outer layer of the implant has oxidized, oxidation of the material at further depths is significantly slowed. Because this layer is important to stemming further oxidation, any damage incurred during implantation or use could significantly reduce the life of the implant (15).
Also responsible for reducing implant life is the galvanic response of the microstructure. As previously stated, cast CoCrMo has a dendritic microstructure with cobalt-poor areas. The difference in electronegativity between adjacent materials sets up an electrochemical reaction, which increases
the rate of internal oxidation (16). Poor thermal treatment can also lead to concentration of chromium in carbides at grain boundaries. This can result in intergranular corrosion and mechanical failure of a device. Annealing after casting or other thermal treatments can eliminate the anodic effects of a dendritic microstructure or grain boundary sensitization to corrosion (17).
the rate of internal oxidation (16). Poor thermal treatment can also lead to concentration of chromium in carbides at grain boundaries. This can result in intergranular corrosion and mechanical failure of a device. Annealing after casting or other thermal treatments can eliminate the anodic effects of a dendritic microstructure or grain boundary sensitization to corrosion (17).
Titanium Alloys
Titanium is used in implants primarily because of its comparatively low density, lower cost, and ease of processing. The mechanical properties of implant-grade titanium alloys, as given by ASTM F136, place the minimum UTS at 860 Mpa and the yield strength at 795 MPa (18). Referring back to the material properties of cobalt alloys, these are roughly equivalent to the annealed, wrought alloy.
Although titanium alloys can have complex phase transformations, engineers are primarily concerned with only the alpha and beta phases of the element. The alpha phase is HCP whereas the beta phase is body-centered cubic (BCC) (12). The existence of the beta phase at room temperature increases density, the heat-treatment response, strength, strain-rate sensitivity, and machinability (19). More alpha phase increases creep strength, allows for thermal treatment such as welding, and prevents crack initiation (20). The preferred structure for titanium alloys in implants is thus equiaxed alpha in an alpha–beta matrix (18). Two common beta-phase titanium alloys are Ti-35Nb-7Zr-5Ta (TNZT) and TiMo12Zr6Fe2 (TMZF), which use zirconium to provide the beta phase (21,22,23).
Mechanical properties for titanium alloys occupy a more constrained range than cobalt alloys, with ultimate strength of approximately 931 MPa and a yield strength of 862 MPa (18). High-cycle fatigue properties are quite good. Cracks tend to initiate at the alpha–beta interface (13), and repeated stress will encourage propagation. The equiaxed microstructure prevents extensive propagation and allows fatigue strengths to range from 500 to 700 MPa (9,13). This compares favorably to cast CoCrMo, which has a fatigue strength of approximately 310 MPa (9).
Titanium nonetheless is subject to fracture (Fig. 12.2), particularly in the presence of a stress concentrator. The notch sensitivity of titanium alloys is higher than cobalt alloys, resulting in numerous design-related device fractures (24) and stress-related device fractures (25).
Like the CoCr alloys, titanium alloys employ passive resistance to slow oxidation. Titanium is a highly electronegative material and converts readily to TiO2 (9). The high electronegativity makes a passive layer so quickly that it ultimately prevents galvanic reactions with other metals (26). As such, unlike in CoCr alloys, anodes and cathodes are not set up in the microstructure and internal corrosion does not occur.
Titanium alloys are nonetheless susceptible to electrochemical degradation (Fig. 12.3). In some cases, the implant may shift cyclically in the body, wearing away the oxide layer (26). This can lead to a much more poor result than expected from
laboratory tests. Furthermore, titanium alloys are subject to “crevice corrosion,” in which an area may concentrate ions that will eventually cause the fluid in the immediate area to be acidic enough to cause additional corrosion (27).
laboratory tests. Furthermore, titanium alloys are subject to “crevice corrosion,” in which an area may concentrate ions that will eventually cause the fluid in the immediate area to be acidic enough to cause additional corrosion (27).
Fixation
Fixation techniques for hip arthroplasty are generally classified as to whether they are natural or artificial in nature. Specifically, devices are currently designed to be fixed through allowing or stimulating bone ingrowth/ongrowth, or they are designed to be cemented in place using a poly-methyl methacrylate (PMMA) bone cement.
The original Charnley low-friction arthroplasty system used a PMMA fixation technique developed by Charnley in the 1960s. Cementing a device in place allows immediate weight transfer from the femoral stem or acetabular cup into the adjacent bone. Charnley described the mode of action as one of grout, as PMMA does not possess any native adhesive properties. Today’s PMMA is quite similar to that used in the 1960s and 1970s. Bone cement is provided to the surgeon in two parts. The powder includes fully polymerized PMMA with benzoyl peroxide to initiate a polymerization reaction, and a dense material to improve radio-opacity (e.g., barium sulfate). The liquid component includes the methyl methacrylate monomer, as well as materials to control the reaction speed and degree of polymerization such as hydroquinone and N,N-dimethyl-p-toluidine.
Mixing the powder and liquid results in the polymerization of the liquid portion with relatively little chemical change in the powder portion. Manufacturers thus work hard to balance the speed of reaction (working time) and mixed viscosity (consistency) against the mechanical properties associated with the final polymerized product. An exploration of all of the factors contributing to the quality and strength of a cement bond is beyond the scope of this chapter. However, it is worth noting that a variety of factors have been identified in the literature that point toward decreased strength in a clinical application. These include high porosity, high levels of radio-opacifiers, high concentrations of antibiotics, or incorrect mixing ratios.
Polymerization of PMMA is a highly exothermic reaction, potentially resulting in a substantial local temperature increase. When a thin cement mantle is used in conjunction with a metal prosthesis, however, the thermal effects are mitigated by the ability of the prosthesis to conduct the heat away from the PMMA and local bone. It has been shown that thick cement mantles or significant penetration into the cancellous bone during surface replacement can lead to thermally induced osteonecrosis (28).
Research in the late 1970s and early 1980s led to the development of bone ongrowth and ingrowth surfaces for hip arthroplasty. It was found that the addition of titanium beads or cobalt beads to the surface of an implant would permit bone ingrowth, depending on the size of the pore structure and the relative motion between the implant and the bone. Ingrowth systems have also included titanium mesh and rough, plasma-sprayed surfaces. Outcomes have been shown to be excellent with these uncemented systems (29), though several recent meta-analyses showing no significant difference in outcomes between cementless and cemented devices (30). The debate has thus turned to one of cost efficacy and value, where studies in Europe suggest that cementless devices place an overall higher cost on the medical system (31).
Newer ingrowth surfaces resembling the natural trabecular structure have been recently introduced, including porous titanium and foamed tantalum. Although it is too soon to identify the long-term clinical outcomes for these devices, shorter follow-up results show excellent performance. The surgeon is cautioned to follow the literature to best understand long-term results and the cost efficacy of these newer ingrowth materials.
Bearing Surfaces
The area of most growth and research in biomaterials for total hip arthroplasty is the bearing couple. Meeting the demands of the modern patient requires interacting surfaces that can withstand high loads, long in vivo durations, millions of cycles, and variation in surgical placement. Although the gold standard remains metal-on-polyethylene, alternative bearings have been introduced over the last four decades, occasionally enjoying substantial market penetration.
Today, over 90% of total hip arthroplasty bearings employ a cobalt alloy articulating against a cross-linked UHMWPE bearing. Additional bearing couples include ceramic-on-polyethylene (CoP), ceramic-on-ceramic (CoC), and metal-on-metal (MoM).
Metal-on-Polyethylene
UHMWPE was first introduced in 1962 as the bearing for the Charnley low-friction arthroplasty (32). Since its introduction, several major changes and advancements have been made to UHMWPE in attempt to improve clinical performance of the total hip replacement.
UHMWPE was initially sterilized using gamma sterilization. Gamma sterilization imparts ionizing radiation on the material, causing scission between carbon–carbon and carbon–hydrogen bonds, leaving open bonding sites known as free radicals (33,34,35). These bonding sites can form some cross-links with nearby chains, however, there is not sufficient energy for all of the bonding sites to form cross-links, and these remaining free radicals are available to participate in a complex suite of self-perpetuating reactions, ultimately leading to oxidation of the polymer (33,36). In the most severe cases, oxidation leads to a dramatic decrease in molecular weight, and a resulting decrease in ultimate mechanical properties. Specifically, the polymer experiences a reduction in strength, ductility, and resistance to fatigue, which renders the bearing surface susceptible to fatigue failure (36,37).
Oxidation is a time-dependent phenomenon, and fatigue failure is cycle-dependent. In fact, gamma-sterilized devices actually have initially improved wear performance over never-irradiated devices because of the presence of molecular cross-links, with performance decreasing with time and the number of articulation cycles. Thus, it is unclear exactly when a device might fail through wear or fatigue failure (Fig. 12.4).
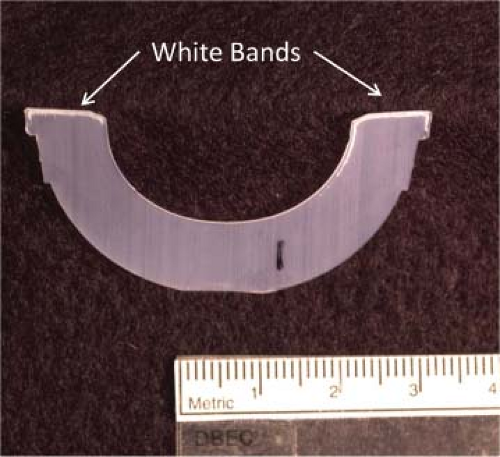
In the laboratory, susceptibility to failure can often be assessed through cross-sectioning a device and performing chemical analysis, though highly oxidized devices quickly reveal themselves visibly through evidence of a “white band” approximately 1 mm below the exposed surface. The white band is an area of higher oxidation and decreased mechanical integrity which is locally cracked during the sectioning process. During in vivo articulation, this region of higher oxidation can be the locus of crack initiation, with propagation continuing to the surface. Large pieces of polyethylene can be liberated, leading to pitting, cracking, and delamination in loaded polyethylene components. Ultimately, large volumes of debris can be released from the articular surface, leading to altered biomechanics and a cascade of cellular signals leading to periprosthetic osteolysis. Retrieval laboratories reported fatigue failure rates approaching 50% for retrieved gamma in air bearings (38), leading to an industry-wide paradigm shift away from using gamma sterilization in air (34,38).
New polymer bearing treatments include sterilization in the absence of oxygen, irradiation and thermal stabilization of free radicals, and irradiation with antioxidant stabilization of free radicals. These treatments are discussed briefly below. For a deeper coverage of the subject, the reader is directed to the excellent handbook on UHMWPE by Kurtz (32).
Changes to Sterilization Methods
The first response to gamma in air sterilization was to eliminate oxygen from the sterilization environment. New techniques were examined, including gamma in an inert gas with barrier packaging (gamma barrier), gamma in a vacuum foil pouch with a vacuum drawn, ethylene oxide gas (EtO) sterilization, and gas plasma sterilization.
The most prevalent option for reducing shelf oxidation of devices was to gamma sterilize devices in an inert gas (such as nitrogen or argon) and use barrier packaging which prevents oxygen diffusion into the package (32). This packaging prevents the UHMWPE from oxidizing on the shelf, however, it has been shown that components sterilized using this method oxidize in vivo because of dissolved oxygen and oxidizing species in vivo (33,38). Figure 12.5 shows that this oxidation can manifest as the aforementioned characteristic white bands in retrieved barrier packaged components (38). Similar to the gamma-air problem, in vivo oxidation reduces the mechanical properties of the UHMWPE, increasing the probability of fatigue failure (25,27,28,29,31).
There are two main alternatives to gamma sterilization: EtO sterilization and gas plasma sterilization. These techniques do not use radiation, and do not cause a chemical
change in the UHMWPE. EtO is a toxic gas that has been used as a sterilization technique since the 1970s (32). Since UHMWPE will not react with the toxic gas, this sterilization technique has been used as an alternative to gamma sterilization (24,26). Studies have shown that EtO sterilization results in little to no oxidation in vivo compared to gamma sterilization (34). In addition, many of the failure modes experienced because of oxidation (delamination, cracking, etc.) are not seen with components sterilized in EtO. However, because of the time-intensive nature of EtO sterilization and the hazardous waste gases involved, it is not always the preferred alternative to gamma sterilization (32).
change in the UHMWPE. EtO is a toxic gas that has been used as a sterilization technique since the 1970s (32). Since UHMWPE will not react with the toxic gas, this sterilization technique has been used as an alternative to gamma sterilization (24,26). Studies have shown that EtO sterilization results in little to no oxidation in vivo compared to gamma sterilization (34). In addition, many of the failure modes experienced because of oxidation (delamination, cracking, etc.) are not seen with components sterilized in EtO. However, because of the time-intensive nature of EtO sterilization and the hazardous waste gases involved, it is not always the preferred alternative to gamma sterilization (32).
Gas plasma sterilization for UHMWPE began in the 1990s during the decline of gamma sterilization in air. Ionized gas such as peracetic acid gas plasma or hydrogen peroxide gas plasma can be used to sterilize the surface of the UHMWPE (32), and it was shown that gas plasma sterilization can improve wear performance compared to a nonsterilized control UHMWPE (35), however, the wear performance is lower than gamma sterilized.
Overall, studies have confirmed that there is minimal risk of introducing free radicals that can bind with oxygen in these two nonradiation methods. However, cross-linking does not occur using either method, and therefore UHMWPE sterilized using EtO or gas plasma sterilization has a greater wear rate than UHMWPE sterilized with gamma radiation (39,40,41).
Most manufacturers now use gas plasma sterilization as the terminal sterilization process, but ethylene oxide sterilization and gamma sterilization using barrier packaging are still used by manufacturers today. Further, the gas plasma process is typically performed after additional treatment to improve the cross-link density, and therefore wear performance of the raw material.
Highly Cross-linked UHMWPE
In the absence of oxidation, the biggest mechanical and materials problem with UHMWPE bearings was the prevalence of osteolysis secondary to wear, resulting in aseptic loosening of femoral and acetabular components (42,43,44). Polyethylene, although biologically inert in the bulk, can cause biologic reactions that cause bone resorption when small wear particles are released into the body. Studies have shown that particles below a micron in size can be phagocytosed, and are therefore the most biologically active (45). Hip joint simulator studies and tissue digestion studies have confirmed that a large number of polyethylene wear particles generated are in the 0.1- to 1-μm range, resulting in osteolysis and subsequent bone resorption (46). Although it was estimated that approximately 0.2 mm/year of wear is sufficient for osteolysis to occur, it is important to recognize that there exists a functional biologic activity for size ranges of particles, and thus smaller particles are more active per unit volume, and thus will result in a more vigorous biologic response for a given wear rate (47).
To decrease the incidence of osteolysis-related revisions, manufacturers pursued methods to reduce the wear rate of bearing surfaces. The most effective defense against wear was shown to be cross-linking of the polymer (32,48). Cross- linking is accomplished by using either gamma radiation or electron beam radiation to break the molecular bonds. Free-radical sites, in the absence of oxygen, are free to reconnect with neighboring chains, thereby increasing the molecular weight of the polymer and forming a highly interconnected network. Although this process is thermodynamically favorable, it is not necessarily immediate, nor complete. Thus, to eliminate or reduce free radicals and promote additional cross-linking, the UHMWPE is heat treated, either above or below the crystalline melt of the polymer to improve mobility of the atoms and therefore improve cross-linking.
The addition of heat has two primary impacts. First, it can speed up hydrogen abstraction and allow free radicals to move from nonreactive sites in crystalline regions to reactive sites in the amorphous regions. This process is one of internal diffusion, and it has been shown that annealing in this fashion can leave residual free radicals. Recent studies suggest that these free radicals oxidize in vivo, potentially leading to polymer damage or delamination in cases of neck impingement or subluxation. To our knowledge, there are no published reports of articular surface delamination of annealed materials in the hip.
The second impact of heat, and in particular, melting, is improved polymer chain mobility. Free radicals on polymer chains have the opportunity to fully recombine or self-annihilate, thereby decreasing the free-radical concentration to below detectable levels. Although the free-radical concentration is substantially decreased during this process, the increased chain interaction within the polymer inhibits recrystallization during cooling. The mechanical properties of the polymer are therefore potentially decreased.
Highly Cross-linked UHMWPE Wear
Cross-linking and thermally stabilizing UHMWPE dramatically reduces wear rates. Wear reduction has been found to be
a function of radiation dose, but it has also been shown that the rate of wear reduction diminishes as the radiation dose increases (49). McKellop et al. showed early on that radiation doses exceeding 100 kGy demonstrated diminishing returns in wear performance (Fig. 12.6). Thus, commercially available remelted material in the US market rarely exceeds a 100 kGy cross-linking dose.
a function of radiation dose, but it has also been shown that the rate of wear reduction diminishes as the radiation dose increases (49). McKellop et al. showed early on that radiation doses exceeding 100 kGy demonstrated diminishing returns in wear performance (Fig. 12.6). Thus, commercially available remelted material in the US market rarely exceeds a 100 kGy cross-linking dose.
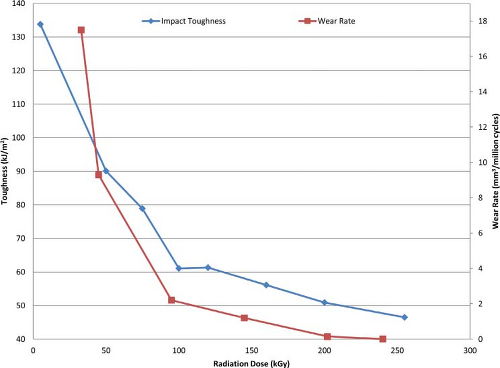 Figure 12.6. Effects of radiation dose on impact toughness and wear rate. (Data from Huot JC, Van Citters DW, Currier JH, et al. The effect of radiation dose on the tensile and impact toughness of highly cross-linked and remelted ultrahigh-molecular weight polyethylenes. J. Biomed. Mater. Res. B. Appl. Biomater.
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|