, James B. Galloway2 and David L. Scott2
(1)
Molecular and Cellular Biology of Inflammation, King’s College London, London, UK
(2)
Rheumatology, King’s College Hospital, London, UK
Abstract
Most emphasis has been placed on the use of tumour necrosis factor inhibitors as the first line biologic agents in inflammatory arthritis patients not responding to DMARD therapy. There are, however, many other biological agents that are effective in DMARD-refractory individuals. Currently five other types of biologics are licensed for the management of inflammatory arthritis. Each drug inhibits a separate pathway involved in arthritis. These other biologics, viewed from the perspective of their mechanisms of action, comprise B-cell inhibition using Rituximab, T-cell modulation using Abatacept, interleukin-6 inhibition using Tocilizumab, interleukin-1 inhibition using Anakinra, and interleukin-12/-23 inhibition using Ustekinumab. This chapter will provide an overview of the mechanisms of action, clinical indications, side-effects and evidence base for each of these agents.
Keywords
BiologicsCytokinesRituximabAbataceptTocilizumabAnakinraUstekinumabIntroduction
Although most emphasis has been placed on tumour necrosis factor inhibitors, there are many other biological agents that are effective in patients with inflammatory arthritis. Currently five other types of biologics are licensed for the management of inflammatory arthritis. Each drug inhibits a separate pathway involved in arthritis.
Two of these biologics affect cellular pathways, which involve either B-cells or T-cells. Three of them inhibit different cytokines. Four are effective in RA and one is effective in PsA. These other biologics, viewed from the perspective of their mechanisms of action, comprise:
B-cell inhibition: Rituximab, which is effective in RA and is given by intravenous infusions.
T-cell modulation: Abatacept, which is effective in RA and can be given by either intravenous infusion or subcutaneous injections.
Interleukin-6 (Il-6) inhibition: Tocilizumab, which is effective in RA and can be given by either intravenous infusion or subcutaneous injections.
Interleukin-1 (Il-1) receptor antagonist: Anakinra, which can be given in RA but has insufficient efficacy to justify its widespread use. Interestingly it is more effective in acute gout and a range of rarer disorders including familial fevers.
Interleukin-12/-23 (Il-12/-23) antagonist- Ustekinumab
Rituximab
Background
Rituximab is a chimeric monoclonal antibody. It depletes the B-cell population by targeting cells bearing the CD20 surface marker [1]. This binding interferes with the activation and differentiation of B cells. It was introduced for the treatment of lymphomas but was subsequently found to be effective in RA. The effect on B-cells suggests that the prevailing view of RA as a predominantly T-cell mediated disease is doubtful.
B Cells in RA
The mechanism or mechanisms by which B cell depletion improves RA are unclear [2]. B cells have potentially important roles in several aspects of RA pathogenesis. Firstly they may act as antigen-presenting cells providing co-stimulatory signals for T cell activation and expansion. Secondly they are involved in the production of rheumatoid factor and anti-cyclic citrullinated peptide antibodies, which lead to immune complex formation and complement activation. These autoantibodies are associated with more severe disease phenotypes. Finally they can be involved in the production of pro-inflammatory cytokines such as TNF and IL-6. These cytokines drive the chronic inflammatory process which characterise active RA.
Mechanism of Action
Rituximab can deplete C20+ B cells by several mechanisms. These include inducing CD20+ B cell lysis by recruiting components of the innate immune system such as macrophages, inducing apoptosis of CD20+ B cells and activating the complement cascade leading to the formation of membrane attack complexes that target CD20+ B cells [2].
Clinical Indications
Rituximab is given in combination with methotrexate to treat severely active RA. Its use is currently focussed on patients who have had an inadequate response or intolerance to standard DMARDs and to one TNF-inhibitor.
Clinical Efficacy
A number of clinical trials have been completed, which all show rituximab reduces joint counts, improves disability and limits joint damage in patients with active RA. It is effective in both treatment naive patients and those refractory to previous DMARDs and TNF-inhibitors. Its use, however, is currently restricted to those with active RA who have failed anti-TNF treatment.
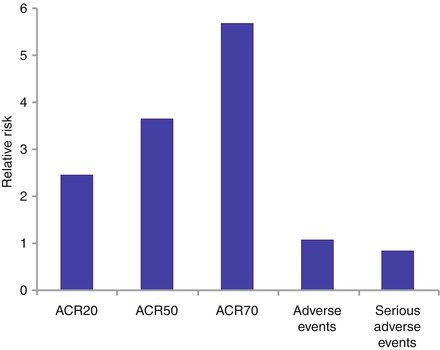
One systematic review has combined findings from three trials, which enrolled 938 patients with active RA [3]. They were all resistant or intolerant to DMARDs or TNF-inhibitors. They were followed up for 6–12 months. Patients receiving rituximab and methotrexate had substantially greater ACR responder rates without more toxicity. These results are summarised in Fig. 9.1
Although it is used in seropositive and seronegative RA patients, the efficacy of rituximab appears to be superior in seropositive individuals. A meta-analysis of four clinical trials identified modest but important clinical benefits in patients who are seropositive for rheumatoid factor and also for anti-cyclic citrullinated peptide antibodies [4]. The reduction at 6 months in DAS28 scores was 0.35 units higher in seropositive patients. The effects of rituximab also mirror rheumatoid factor levels. Serum autoantibody levels fall in association with clinical responses.
Initial and Repeat Courses
A course of rituximab comprises two 1 g intravenous infusions 2 weeks apart given alongside intravenous methylprednisolone. It is given with methotrexate, which potentiates its efficacy and durability. A single course of treatment results in a near total peripheral blood B cell population depletion. This usually persists for 6–9 months. CD20 is expressed on only pre-B cells and mature B cells; it is not expressed on stem cells or plasma cells. Therefore B cell repopulation occurs after this time point by naive B cells.
In patients who respond further infusions can be given at intervals of at least 6 months. The optimal time to give repeated infusions is not certain. Currently practiced regimens comprise:
1.
Regular re-treatment: every 6-months
2.
Treat-to-target: re-treat every 6 months until remission is achieved
3.
Re-treatment on flare: an approach undertaken in early clinical trials
Although trial data indicates a treatment-to-target strategy may be best, limited long-term safety data in RA indicates the need for caution with regular re-treatment.
Adverse Reactions
Most adverse effects are infusion-related reactions, and are generally mild to moderate. Reactions to the first infusion such as hypotension and fever are common, occurring in one third of patients. They are reduced by corticosteroid prophylaxis with intravenous methylprednisolone. Such reactions are usually reversed by reducing or interrupting the infusion alongside therapeutic intervention with paracetamol and antihistamines. They are less common with repeated courses. Severe infusion reactions leading to drug discontinuation are rare occurring in less than one percent of patients.
A range of other adverse events may occur [5], and these have been studied in a cohort of 3,194 RA patients who had received up to 17 rituximab courses over 9.5 years. Rituximab remained well tolerated over time and multiple courses. There was no indication of an increased safety risk with rituximab over time.
Infection
There is a slightly increased rate of infection in RA patients receiving rituximab. Data from the biologics registries indicates that serious infections are greatest in the first few months following treatment. As with all biologics rituximab should be avoided in patients with an active infection or those who are severely immunocompromised.
Unlike anti-TNF therapy the risk of TB does not appear increased and there is no current evidence indicating the need to screen RA patients for TB prior to rituximab treatment.
Hepatitis B virus reactivation has been well documented in oncology patients, but not RA patients treated with rituximab. In patients with serological evidence of previous or current hepatitis B infection the risk-benefit profile should be fully discussed. If rituximab is deemed necessary then patient management should be undertaken in consultation with a gastroenterologist. Prophylactic treatment against hepatitis B reactivation with lamivudine may be considered. Current practice is to pre-screen patients for previous/current hepatitis B infection. The risk of hepatitis C virus is unclear although there are reports of severe infusion reactions in hepatitis C infected patients. It is recommended that patients should be screened for risk factors for hepatitis C pre-treatment.
To date a few cases of progressive multifocal leukoencephalopathy have been reported in RA patients. Most had long-standing RA with previous use of multiple immunosuppressive agents. Progressive multifocal leukoencephalopathy is caused by reactivation of the common JC virus. It occurs in immunocompromised individuals, resulting in myelin loss within the nervous system. Symptoms are protean and depend upon the location and degree of myelin loss. They include personality changes, visual disturbances and weakness. Although the risk is very small (currently estimated at 1 in 20,000) its poor prognosis and lack of specific treatment means that patients should receive pre-treatment counselling regarding it.
Anti-biologic Antibodies
Human anti-chimeric antibodies are detected in about 10 % of patients and may be associated with worse infusion or allergic reactions and failure to deplete B-cells after subsequent courses.
Tocilizumab
Background
IL-6 is an important pro-inflammatory cytokine in RA. It promotes inflammation through the expansion and activation of T cells, differentiation of B cells and induction of acute-phase reactants by hepatocytes. IL-6 signal transduction is mediated by membrane-bound and soluble receptors [6]. Tocilizumab is at present the only available IL-6 inhibitor for the treatment of inflammatory arthritis. It is a recombinant humanised anti-human IL-6 receptor monoclonal antibody of the IgG1 subclass. It binds to both membrane-bound and soluble IL-6 receptors, preventing their activation by IL-6.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree