15
Autologous Chondrocyte Implantation
Full-thickness chondral lesions in the knee have been known to be problematic for over two centuries.1 The incidence and natural history of articular cartilage lesions have not been well defined; however, a lesion in the weight-bearing surface of the knee may progress to osteoarthritis. This is especially thought to be true for larger lesions that are unshouldered. Frequently, chondral injuries are identified incidentally at the time of arthroscopy for the treatment of meniscal or ligamentous injuries.2,3 Articular cartilage is a unique tissue with limited potential for repair because it lacks vascular, lymphatic, and nerve supply. Subsequently, symptomatic articular cartilage lesions have been difficult management problems for the orthopaedist.
Total knee arthroplasty has proven to be a reliable and durable procedure. However, prostheses begin to fail at an increased rate over the second decade of their use. Thus, young patients who undergo total knee arthroplasty may be subjected to multiple revision surgeries with less predictable results.4
There are a variety of treatment options for the orthopaedist treating chondral injuries of the knee. Arthroscopic debridement, or marrow-stimulating techniques including abrasion chondroplasty, microfracture, and drilling may be useful for the patient with symptomatic chondral injuries. Additionally, osteochondral allografting, osteochondral autografting by open or arthroscopic techniques, and autologous chondrocyte implantation have been used to manage the symptomatic patient with chondral injuries. Patient factors as well as the characteristics of the chondral injury including its size, location, and depth help determine which method is most suitable for a particular patient. Each of these treatments may provide some benefit to properly selected patients. Marrow-stimulating techniques produce a fibrocartilage repair tissue, which has been found to have inferior mechanical properties compared with hyaline cartilage.5 Only osteochondral autograft/allograft techniques and autologous chondrocyte implantation produce a hyaline cartilage repair tissue. Autologous chondrocyte implantation may lessen the intraarticular donor-site morbidity associated with osteochondral graft transfers and eliminates the concerns of using an allograft if one chose to use an allograft technique. Additionally, the technique may allow for a more homogeneous repair surface compared with the potential “cobblestone” repair surface obtained with osteochondral autograft as per the mosaicplasty technique.
Autologous chondrocyte implantation has produced hyaline repair tissue in experimental models as well as in biopsies taken from second-look arthroscopies,6 and this technique has produced durable results in a midterm, 2- to 9-year, follow-up report of patients in Sweden. Encouraging results also have been shown at short-term follow-up in a series of patients in the United States.7 Interestingly, in this series, many of the patients who were significantly improved had multiple or complex cartilage lesions. In select patients, autologous chondrocyte implantation has the benefit of providing end-differentiated chondrocytes to large areas of the injured articular surface. By resurfacing these areas of chondral injury with hyaline cartilage, it is hoped this technique will resolve symptoms and facilitate a return to an active lifestyle for these young patients. Furthermore, the progression of osteoarthritis may be halted or delayed.
 Healing Process of Autologous Chondrocyte Implantation
Healing Process of Autologous Chondrocyte Implantation
Many studies have been performed that explore the healing process of autologous chondrocyte implantation. Rabbit studies have been performed that demonstrate the in vitro cultured chondrocytes to be responsible for the majority of the in vivo repair tissue, and this repair tissue surpassed periosteum alone in quality and quantity.8,9 Canine studies have revealed that the healing process has distinct phases of healing.10,11 These stages have been divided into the proliferative stage (0 to 6 weeks), the transition stage (7 to 12 weeks), and a remodeling and maturation stage, which occurs over an extended time frame (13 weeks to 3 years)12 (Table 15–1).
During the proliferative stage, a primitive cell response occurs with tissue fill of the defect. At this time, the tissue has poor integration to the underlying host bone or adjacent cartilage. This tissue is soft and jelly-like, mostly composed of type I collagen and easily damaged at this stage.12 The transition phase is marked by the production of a type II collagen framework. Proteoglycans are produced and imbibe water, giving the articular cartilage its viscoelastic properties. At this stage, the tissue has the consistency of firm gelatin and is milkable when probed arthroscopically, demonstrating that it is not yet well integrated into its surrounding tissues.12
During the stage of remodeling and maturation, the collagen matrix is stabilized, by cross-linking, to the underlying bone and adjacent cartilage. At 4 to 6 months, the tissue is integrated to the underlying bone and has a putty-like consistency. It is at this point that patients begin to experience good symptom relief, and they discard their assistive devices. However, the mechanical properties at 4 to 6 months are not yet optimized. It is at this point when symptoms are resolving that excessive activity may cause repair tissue degeneration. Ultimate maturation and stabilization of the repair tissue occurs between 1 and 3 years, depending on patient factors such as patient age and defect size and location.12
 Patient Presentation and Assessment
Patient Presentation and Assessment
Clinical Evaluation
Patients with articular cartilage injuries may present with a variety of symptoms, including prior traumatic injuries, pain, effusion, locking, catching, malalignment of the extremity, and patellar maltracking. To accurately assess if a patient may be a candidate for autologous chondrocyte implantation, a thorough history and physical examination are essential. Prior traumatic injuries are common, and in the senior author’s experience, patients who have had anterior cruciate ligament (ACL) injuries are particularly predisposed to chondral injuries. Johnson13 has noted that 20% of ACL injured knees chronically develop full-thickness cartilage loss. Furthermore, full-thickness chondral injuries have an incidence of between 5% and 10% in patients who have experienced acute work-related or sporting injuries and present with an acute hemarthrosis.2
Pain is a frequent complaint, and the location of the pain can help localize the site of chondral injury. Medial or lateral joint line pain focuses attention on the medial or lateral compartments. Locking or catching of the knee may represent the presence of a loose body from an osteochondral injury. These symptoms also may represent a meniscal injury, and this possibility needs to be assessed prior to embarking upon treatment of the chondral injury. Meniscal pathology can then be treated by meniscal debridement, meniscal repair, or meniscal transplantation if it exists at the time of surgery.
Recurrent effusions are also associated with full-thickness chondral injuries. This is particularly true in the case of trochlear lesions. Additional complaints focusing attention on the patellofemoral articulation include anterior knee pain when ascending or descending stairs or inclines, as well as anterior knee pain with prolonged sitting. Pain that is noted in the posterior aspect of the knee also may be related to patellofemoral pathology. Causes of the patellofemoral pathology may be due to prior dislocations or patellar maltracking, resulting in full-thickness chondral injuries. Clinically, evaluation should include evaluation of the Q-angle, hypermobility of the patella, and pain with a patellar grind test. Maltracking must be addressed if one is considering autologous chondrocyte implantation (ACI) to this articulation. Early failures of ACI may have been related to the immature cells continuing to be exposed to increased shear forces due to the maltracking.6
Similarly, axial malalignment may also accompany full-thickness chondral weight-bearing injuries. Clinical evaluation may include the observance of genu varum or valgum and the presence of a lateral thrust during gait. However, long axial alignment x-rays should always be performed to confirm or exclude the clinical impression of alignment. As with the patellofemoral joint, any consideration of ACI must first include correction of any axial malalignment to avoid exposing the immature chondrocytes to increased forces from a malaligned extremity. The decision to perform the realignment procedure concurrently with ACI, or in a staged sequence with the realignment procedure followed at a later date by ACI, is made on a case—by-case basis taking into account individual patient factors.
Ligamentous stability may be just as important to the knee as correct axial alignment when considering ACI as a treatment for a patient with a full-thickness chondral injury. Performing ACI without restoring joint stability may result in the graft site experiencing increased shear forces from recurrent episodes of instability, predisposing the graft to failure. As with realignment procedures, the decision to perform ligament reconstruction concurrently with ACI or in a staged fashion is determined on a case-by-case basis.
Radiographic Evaluation
After obtaining an appropriate history and physical exam, the clinician must then request a radiographic evaluation, which is necessary to diagnose an articular cartilage injury and determine if a patient is a candidate for ACI. A standard radiographic protocol should include weight-bearing anteroposterior and lateral radiographs to assess joint space narrowing and osteophyte formation. Rosenberg et al14 suggest that standing 45-degree posteroanterior views are also helpful in diagnosing tibiofemoral cartilage loss or osteochondral defect lesions. Additionally, standing 54-inch axial alignment views that include the center of the femoral head and talus are essential to assess the overall axial alignment of the extremity and determine if realignment will be required. Merchant, or sunrise, views assess the patellofemoral articulation for evidence of joint space narrowing or maltracking. Select cases may require a computed tomography (CT) scan of the knee with the leg in extension and the quadriceps contracted, and relaxed, to better assess patellofemoral tracking. Merchant or sunrise views may not reveal some cases of maltracking because the patella engages the trochlea with knee flexion. CT also reveals the rare case of a hypoplastic trochlea that may contribute to patellar maltracking or obvious subluxation. Failed proximal patellar realignment procedures often occur because of unrecognized trochlea dysplasia.
Magnetic resonance imaging (MRI) allows visualization of the articular cartilage as well as the menisci, ligaments, and bone, making it a valuable tool in evaluating patients with suspected articular cartilage injuries. However, technical issues mainly related to spatial resolution and volume averaging can make it difficult to strictly determine exact dimensions of cartilage defects or repair tissues.15 Newer imaging techniques and protocols have improved the accuracy of evaluating cartilage lesions and repair tissues, and MRI now is a valuable tool in the evaluation of patients with articular cartilage lesions. Winalski and Minas15 note that the techniques representing state-of-the-art clinical evaluation of articular cartilage include fat-suppressed three-dimensional (3D) T1-weighted gradient echo images, fast spin echo sequences, and magnetic resonance arthrography (MRA). These techniques have improved the sensitivity of detecting cartilage injury to over 85% for moderate to severe cartilage lesions (Outerbridge grade 2–4)16 while maintaining a specificity of over 90%.15 However, the availability of these techniques may be limited to specialized centers.
Fat-suppressed 3D T1-weighted gradient echo images cause the articular cartilage to appear bright compared with the surrounding joint fluid and subchondral bone and bone marrow.15 This produces a very accurate representation of the cartilage-fluid and cartilage bone interfaces,17 and is therefore a very good method for determining articular cartilage defects. Unfortunately, this technique does not adequately assess the menisci or ligaments.15
Fast spin echo techniques facilitate both improved image contrast between cartilage and fluid and assessment of the subchondral bone.15 Additionally, this technique facilitates evaluation of the menisci and ligaments and minimizes metallic artifact. However, the images are prone to blurring and partial volume averaging inaccuracies.15
Magnetic resonance arthrography (MRA) may be performed either directly with intraarticular injection of contrast or indirectly through intravenous administration of contrast.15 It has been shown to be an excellent method for articular cartilage evaluation.18 When using indirect arthrography, it is imperative to exercise the joint for a period of time prior to imaging to ensure optimal joint fluid enhancement.15
Arthroscopic Evaluation
Traditionally, arthroscopy has been the gold standard when evaluating a patient with a suspected articular cartilage injury, and it continues to play a critical role in evaluating and planning treatment. Arthroscopic evaluation and cartilage biopsy requires a careful and systematic approach. Furthermore, arthroscopy has the benefit of facilitating a staged procedure by harvesting a cartilage biopsy; it also facilitates potential treatment of the cartilage injury, depending on the lesion characteristics. The treatment should be discussed with the patient pre-operatively.
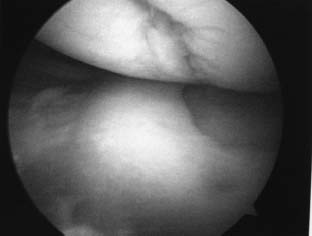
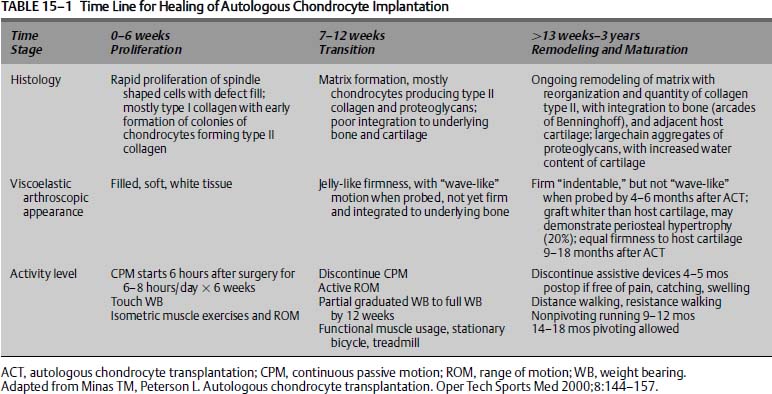
FIGURE 15–1 Arthroscopic photograph of a symptomatic full-thickness chondral injury of the medial femoral condyle.
The arthroscopic examination should begin with an examination of the knee under anesthesia, noting the range of motion and any ligamentous insufficiencies. An arthroscopic probe should be used to determine the extent of grade 3 and 4 chondromalacia16 (Fig. 15–1). The opposing articular surface should also be examined to evaluate if there is cartilage damage on this surface, as ACI has traditionally been reserved for unipolar lesions with no more than grade 2 chondromalacia16 on the opposing articular surface. The dimensions of the lesion(s) should be recorded to facilitate future planning by obtaining an appropriate volume of cultured chondrocytes. One vial of cultured autologous chondrocytes has a volume of 0.4 mL corresponding to 12 million cells, and this may cover a defect size of about 4 to 6 cm2.12 Additionally, a spinal needle can be used to assess the depth and quality of the surrounding cartilage as the bevel of the needle is about 3 mm. This is important for planning the ACI procedure itself, for periosteum will need to be sutured to synovium or through drill holes in the bone if uncontained defects are present.12 Intraoperative examination of the menisci and ligaments is also important to anticipate the future need for ligamentous repair or possible meniscal allograft transplantation.
If a chondral lesion is found to be appropriate for ACI, surgeons should be prepared to perform a cartilage biopsy, which should be discussed with the patient preoperatively. Loose bodies that may be retrieved during arthroscopy typically are not satisfactory for cell culturing, unless they are large osteochondritis dissecans (OCD) fragments. Biopsies may be performed from the superior medial edge of the trochlea, the superior transverse trochlear margin adjacent to the suprapatellar synovium, or the lateral intercondylar notch.12 We choose to perform the biopsy in the superior and lateral intercondylar notch staying anterior to the sulcus terminalis on the lateral condyle (Fig. 15–2). This enables the surgeon to perform the biopsy without compromising a weight-bearing surface. The patellofemoral joint also is protected from being involved in the harvest, as the patella typically does not engage the intercondylar notch before 120 degrees of knee flexion. When performing the biopsy, the patient’s knee is brought through a range of motion, ensuring that the proposed biopsy site is not involved in tracking of the patellofemoral articulation. We then score the proposed biopsy site with an arthroscopic gouge and elevate the biopsy specimen with a twisting motion, bracing the operative hand against the patient to avoid skiving. The biopsy specimen is then removed. The biopsy should consist of 200 to 300 mg of articular cartilage (about 5 mm wide by 10 mm long), as this is the amount required for enzymatic digestion and cell culturing.12 This specimen contains about 200,000 to 300,000 cells, which is enzymatically digested and grown to about 12 million cells per 0.4 mL of culture media per implantation vial.12 In vitro expansion of cells occurs over 3 to 5 weeks and is then suitable to accommodate a defect size of 4 to 6 cm2 per implantation vial.12
FIGURE 15–2 Biopsy performed at the superolateral aspect of the intercondylar notch.
 Indications
Indications
Autologous chondrocyte implantation has been in use in Sweden since 1987.19 Since the initial report on this technique in the New England Journal of Medicine in 1994,20 ACI has become a recognized technique for the treatment of chondral injuries of the knee. Based on this initial study, the classic indications for ACI have been reserved for symptomatic patients with isolated Outerbridge grade 3 to 4 lesions of the femoral condyle or trochlea in patients between the ages of 15 and 55 with no more than grade 1 to 2 chondromalacia on the opposing articular surface.19 Osteochondritis dissecans of the medial or lateral femoral condyles has also been an accepted indication. ACI has not been traditionally indicated for kissing lesions or for patients with radiographic evidence of joint space narrowing.
Two reports noted that these isolated lesions are uncommon.3,7 However, the senior author’s experience has extended the indications to include patients with multiple lesions and “salvage” cases with early arthritic change. The results have been encouraging at 2- to 7-year follow-up.7
 Patient Selection
Patient Selection
Currently, our approach to treatment includes the findings of the clinical exam, plain radiographs, MRI, and an understanding of the patient’s goals and desires (Fig. 15–3). This enables us to match an appropriate treatment plan with the patient’s expectations to ensure the optimal outcome. Arthroscopy is then used to further evaluate the suspected chondral defect. In general, small grade 3 or 4 lesions (<1.5 cm2) are treated with a chondroplasty technique in relatively sedentary patients. Lesions of similar size in very active athletic patients are more likely to be treated with a microfracture or an osteochondral autograft transplantation system (OATS)™ (Arthrex, Naples, FL) technique using a single plug taken from the periphery of the lateral condyle anterior to the sulcus terminalis to limit donor-site morbidity.
Autologous chondrocyte implantation is used for grade 3 to 4 symptomatic lesions of the femoral condyles, trochlea, and patella when the lesions are 2 cm2 or larger or when previous treatment methods have failed. As previously discussed, we carefully examine patients for malalignment, ligamentous insufficiency, and meniscal pathology. We treat patients with these coexisting conditions with staged or combined procedures, depending on patient preferences.
In addition to coexisting orthopaedic issues, general medical comorbidities such as cigarette use and the use of medications that may impair cell proliferation such as nonsteroidals or immunosuppressives are investigated. We do not proceed with ACI until the patient is free from nicotine, as it has been found to impair healing in certain conditions.21,22 Furthermore, patients frequently are taking narcotic pain medications upon presentation due to numerous prior surgeries. We require patients to eliminate narcotic medications prior to surgery to enhance postoperative pain management. If the patient is unable to eliminate nicotine or requires these other medications, other treatment alternatives are considered.
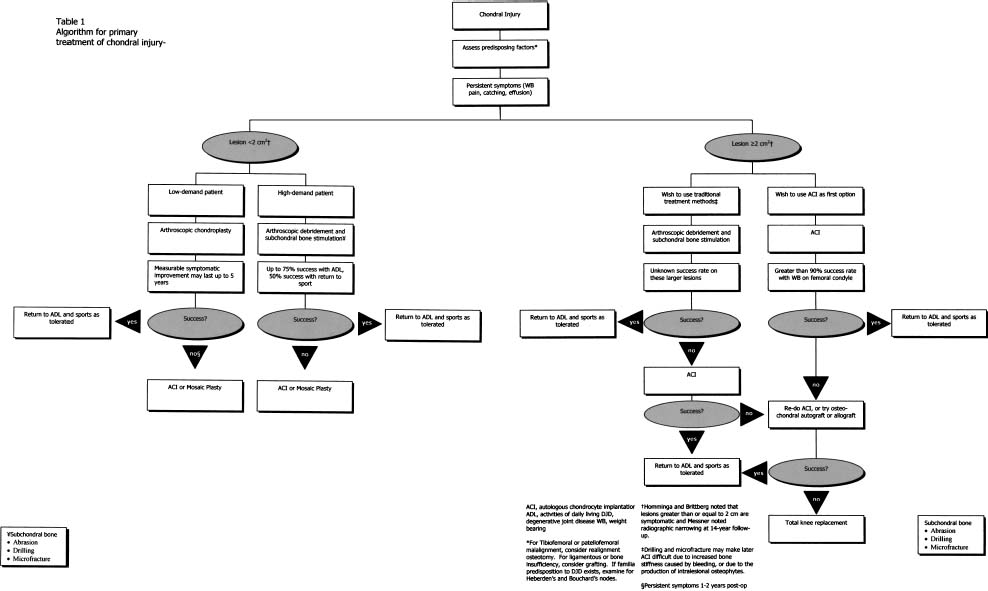
FIGURE 15–3 The senior author’s algorithm for the treatment of patients with full-thickness chondral injuries. (Adapted from Minas T. The role of cartilage repair techniques, including chondrocyte transplantation, in focal chondral knee damage. In: American Academy of Orthopedic Surgeons, Zuckerman JD, ed. Instructional Course Lectures, vol. 48. Rosemont, IL: 1999:629–643)
Malalignment is treated with corrective osteotomy. When addressing tibiofemoral malalignment, we aim to restore the mechanical axis to neutral, if there is no evidence of joint space narrowing. We plan for the weight-bearing axis to pass through the lateral tibial spine for patients with a varus deformity if joint space narrowing exists or peripheral osteophytes are present. Similarly, the weight-bearing axis is planned to pass through the medial tibial spine for a valgus deformity in the presence of joint space narrowing or peripheral osteophyte formation. Having the weight-bearing axis pass through the tibial spine results in roughly a 2- to 4-degree overcorrection, thus unloading the affected compartment. We will also overcorrect malalignment in patients with an absent meniscus or when a large (>10 cm2) weight-bearing lesion is transplanted. The degree of correction is based on our preoperative assessment of angular deformity from the 54-inch films.
Patients with patellar or trochlear lesions typically have associated patellar maltracking and are treated most commonly with a lateral release, medial advancement of the vastus medialis, and possibly a tibial tubercle osteotomy. We have found the Fulkerson23 tibial tubercle osteotomy to be a very effective osteotomy, as it can correct the maltracking and unload the joint by elevating the tibial tubercle.
Anterior cruciate ligament deficiencies are corrected prior to ACI, as knee instability is detrimental to graft survival. Similarly, patients who have undergone previous subtotal meniscectomy may be considered for allograft meniscal transplantation. Attending to these comorbidities should improve the success of ACI by protecting the graft during the healing stages, and help prevent the progression of degenerative changes.
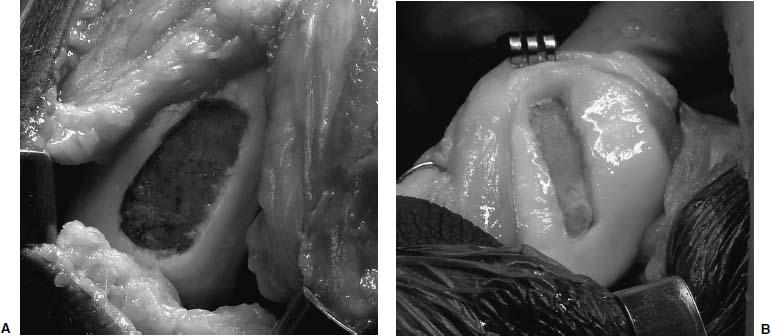
FIGURE 15–4 Multiple chondral lesions may be present. Medial femoral condyle (A) and patellar lesions (B) after debridement to stable edges.
In the senior author’s practice, it is uncommon to have patients with isolated defects. Many patients are found to have multiple defects, and it is not uncommon to place ACI grafts at more than one site at the same surgery (Fig. 15–4). Some young patients have opposing chondral defects. These “kissing” lesions frequently involve the patella and trochlea, and less frequently involve the femur and tibia. These young patients are treated very aggressively with osteotomy as well as ACI to these multiple sites. Additionally, they may have evidence of early arthritic change including joint space narrowing.
Patient education is critical to a successful outcome. There may not be a good treatment option for this type of patient, but in our practice an ACI is considered, provided the patient has a thorough understanding of the rehabilitation process and the potential complications, and desires a biologic approach to the problem. Extensive preoperative counseling is required with these patients to involve them in the decision-making process, for failure rates and complications such as graft hypertrophy and arthrofibrosis may be higher in these salvage cases. If ACI does not appeal to a patient or if it fails, unicondylar knee replacement or patellofemoral arthroplasty is an alternative, as is salvage treatment. Total knee arthroplasty is reserved for tricompartmental disease.
 Surgical Technique
Surgical Technique
Autologous chondrocyte implantation is performed as a separate procedure after cartilage biopsy and cell expansion have occurred. Preoperative antibiotics are administered, and a tourniquet is usually used during preparation of the graft site and harvesting of the periosteum. A bump is used to allow knee flexion of 90 to 100 degrees, and a post is placed on the lateral aspect of the thigh to allow for a stable extremity when suturing the periosteal graft.
A midline incision is utilized to avoid wound-healing issues if and when future surgeries are indicated. Alternatively, two incisions may also be used—the proximal for the arthrotomy and the distal for the periosteal harvest (Fig. 15–5). Full-thickness medial and lateral skin flaps are raised, thus facilitating adequate exposure when concurrent osteotomy is required. A standard medial parapatellar arthrotomy is performed to access the joint, unless an isolated lateral femoral condylar defect is present warranting a lateral arthrotomy. When previous arthroscopy reveals a lesion that extends to the posterior aspect of the condyle, a meniscal takedown is required to facilitate adequate visualization and debridement of the lesion. The coronary ligament is sharply incised along with the anterior-medial attachment site of the meniscus. A full-thickness soft tissue sleeve is then elevated in an anterior to posterior direction, providing excellent exposure of the posterior aspect of the femoral condyle.
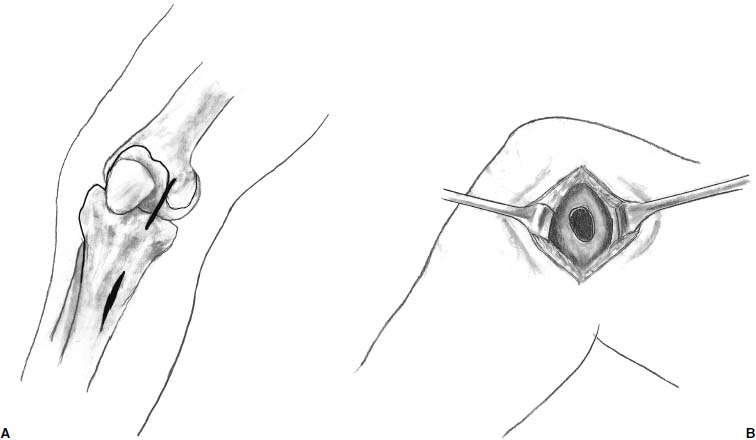
FIGURE 15–5 A midline incision or two medially based gain access to the joint.
Radical debridement of the chondral lesion is crucial to the success of ACI (Fig. 15–6). Debridement is performed back to a stable edge of intact articular cartilage with vertical edges (Fig. 15–7). Occasionally, lesions are uncontained, with no peripheral margin of intact articular cartilage. In these instances, the periosteum needs to be sutured to synovium or through small drill holes through bone to contain the cell suspension. In cases where previous marrow stimulating techniques have been performed, there are frequently intralesional osteophytes that must be debrided. Similarly, lesions associated with osteonecrosis have sclerotic bases that must be debrided to viable bone without violating the subchondral bone plate. These debridements are best performed with the use of a high-speed burr. Bone grafting is required if the depth of the lesion is >10 mm; this may be performed either as a staged procedure or during the same setting as the ACI.
After the lesion has been debrided, it is templated most easily with a marking pen to outline the size of the lesion (Fig. 15–7). The wrapping from the surgical gloves is then placed over the lesion and the ink is transferred onto the paper (Fig. 15–8). The proper orientation of the lesion (12 o’clock position) is marked on the paper. The template is then cut from the glove wrapping about 2 mm larger than the markings (Fig. 15–9). A thrombin-and epinephrine-soaked sponge is then placed over the prepared defect to limit bleeding when the tourniquet is deflated (Fig. 15–10).
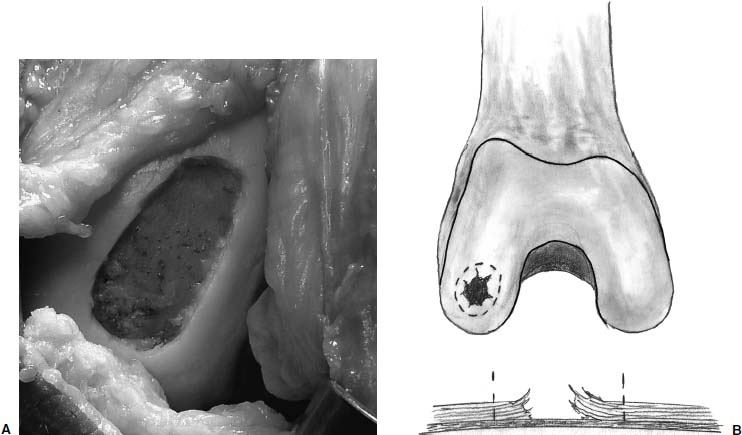
FIGURE 15–6 (A,B) Debridement is performed back to a stable edge of intact articular cartilage with vertical edges.
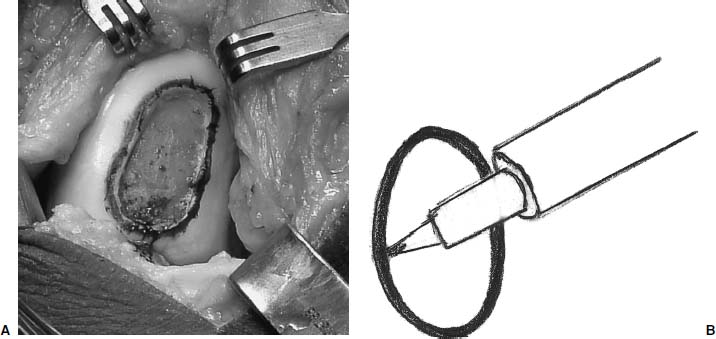
FIGURE 15–7 (A,B) The lesion is outlined with a marking pen.
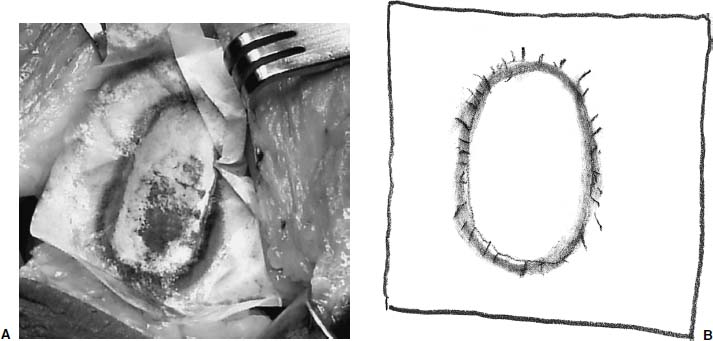
FIGURE 15–8 (A,B)The wrapping from the surgical gloves is then placed over the lesion and the ink is transferred onto the paper.
The periosteum is then harvested using a second incision over the medial tibia, distal to the pes anserine insertion. Fat is cleared from the periosteum, and small retractors facilitate exposure of the tibia. The periosteum is sharply incised 1 to 2 mm larger than the prepared template (Fig. 15–11A). One must remember to mark the periosteum with a marking pen, similar to the template, to ensure optimal orientation of the periosteum and superficial surface prior to suturing. With large lesions, multiple lesions, or revision surgeries, it may be difficult to obtain an adequate amount of periosteum from the medial aspect of the tibia. In these cases, additional periosteum can be harvested from the distal medial or lateral aspect of the femur, though this may increase the risk of arthrofibrosis. Smooth forceps and a sharp periosteal elevator are then used to elevate the periosteum (Fig. 15–11B). If the graft is perforated, the defect is closed with suture when it is placed over the cartilage lesion.
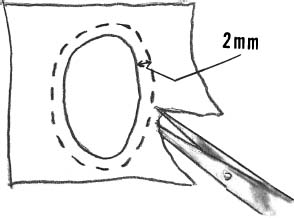
FIGURE 15–9 The template is then cut from the glove wrapping about 2 mm larger than the markings.
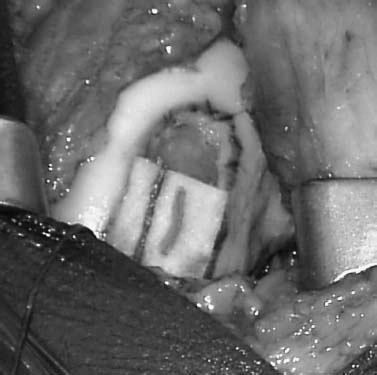
FIGURE 15–10 A thrombin and epinephrine soaked sponge is then placed over the prepared defect to limit bleeding.
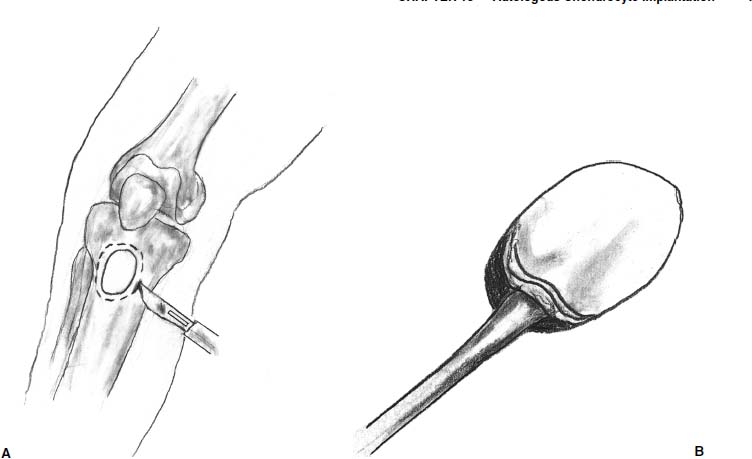
FIGURE 15–11 (A) The periosteum is sharply incised 1 to 2 mm larger than the prepared template. (B) A sharp periosteal elevator is then used to elevate the periosteum.
After harvesting the graft, the tourniquet is deflated and hemostasis achieved in the soft tissues and in the prepared defect. The graft is then placed in its correct orientation with the cambium layer facing the defect. Suturing is then performed, at 3- to 5-mm intervals, with 6-0 Vicryl sutures that have been soaking in sterile mineral oil (Fig. 15–12). An opening is left to allow insertion of a catheter to test graft integrity and allow injection of the cell suspension (Fig. 15–13). Fibrin glue is then placed over the edges of the graft to obtain a watertight seal (Fig. 15–14). Sterile saline is injected into the defect to ensure the watertight seal. The saline is then aspirated out of the defect, and the cell suspension is then injected into the defect (Fig. 15–15). Finally, the opening at the catheter insertion site is sealed with suture and fibrin glue.
If meniscal takedown was required, the knee is extended and the meniscus reduced. Number 2 braided, nonabsorbable suture is placed through the anteriormedial attachment of the meniscus and is anchored through bone. Suction drains are not placed intraarticularly to avoid injury to the graft. The wound is then closed in layers with a drain in the subcutaneous tissues if a lateral release was performed.
 Postoperative Care
Postoperative Care
A knee immobilizer is used to keep the leg in extension for the first postoperative day to allow the chondrocytes to adhere to the underlying subchondral bone and surrounding articular cartilage. Following this, the postoperative rehabilitation is guided by the three healing phases of ACI as identified in canine studies (Table 15–1 and Fig. 15–16).10–12 The principles of rehabilitation are to protect the graft, retain knee motion, and gradually progress activity and weight bearing. The rehabilitation process is somewhat different for lesions of the patellofemoral joint and lesions of the tibiofemoral joint.
For lesions involving weight-bearing areas of the femoral condyles, rehabilitation involves continuous passive motion (CPM) 6 hours a day,24 with a goal of 0 to 90 degrees. Touchdown weight bearing is allowed and isometric exercises encouraged during the first 6 postoperative weeks.
Continuous passive motion is discontinued and graduated weight bearing is initiated during postoperative weeks 7 to 12. By 12 weeks, full weight bearing status is attained, provided that the patient is free of pain and functional muscle usage is encouraged. Stationary bicycling, without resistance, is also helpful during this time.
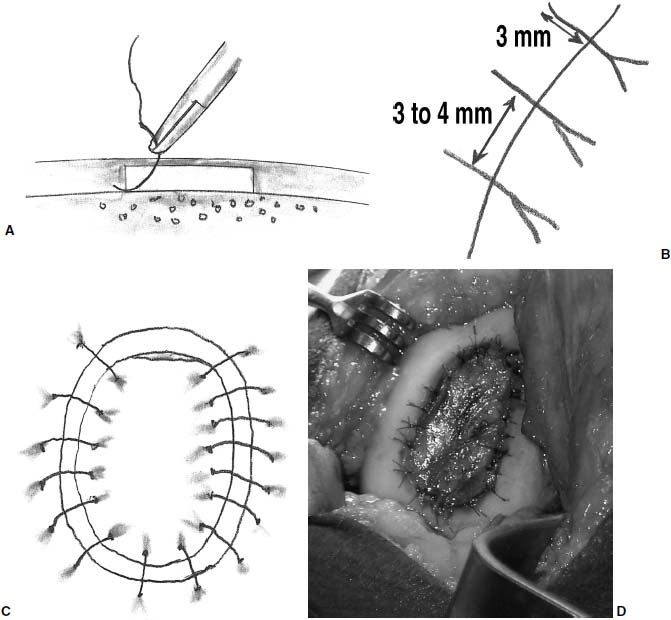
FIGURE 15–12 Suturing is then performed (A), at 3- to 5-mm intervals (B), all around the lesion (C). Full appearance of Sutured periosteal cover prior to cell injection under the periosteum, and sealing margins with fibrin glue (D).
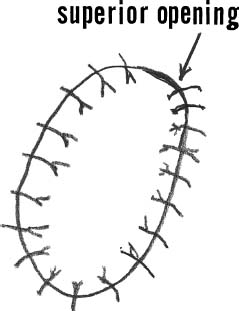
FIGURE 15–13 An opening is left at the top for insertion of a catheter for injection of the cell suspension.
The maturation phase of healing begins after 12 weeks and can extend to 2 years postoperatively. During this time, assistive devices are discarded and activity levels are increased. Bicycling continues to be encouraged as well as the use of elliptical training machines and treadmill walking. Patients should be encouraged to take an active role in their rehabilitation, and they can begin to use resistive training with the stationary bike and treadmill. Running is not permitted until graft hardness is similar to that of the surrounding cartilage, which may take 12 to 24 months.12 Activity should be encouraged that fatigues the thigh musculature, but patients should be counseled to lessen their level of activity if they feel knee discomfort.
When ACI is performed on the patellofemoral joint, the rehabilitation differs. These lesions typically rehabilitate at a slower pace than lesions of the femoral condyles. Continuous passive motion is used for a range of 0 to 40 degrees during the initial 6 postoperative weeks and full weight bearing is allowed with the knee in full extension. This avoids the maximal contact stresses of the patellofemoral joint that occur between 40 and 70 degrees of flexion. The rest of the motion is gained by dangling the leg over the edge of a bed. Isometric straight leg lifts are instituted postoperatively. Active flexion and passive knee extension is encouraged for the first 6 weeks. Backward treadmill walking, elliptical training machines, and stationary bicycling with minimal resistance are allowed between 6 and 12 weeks. Persistent effusions are common for up to 6 months with trochlear lesions. Kneeling and squatting activities may be allowed 12 to 18 months after surgery.12
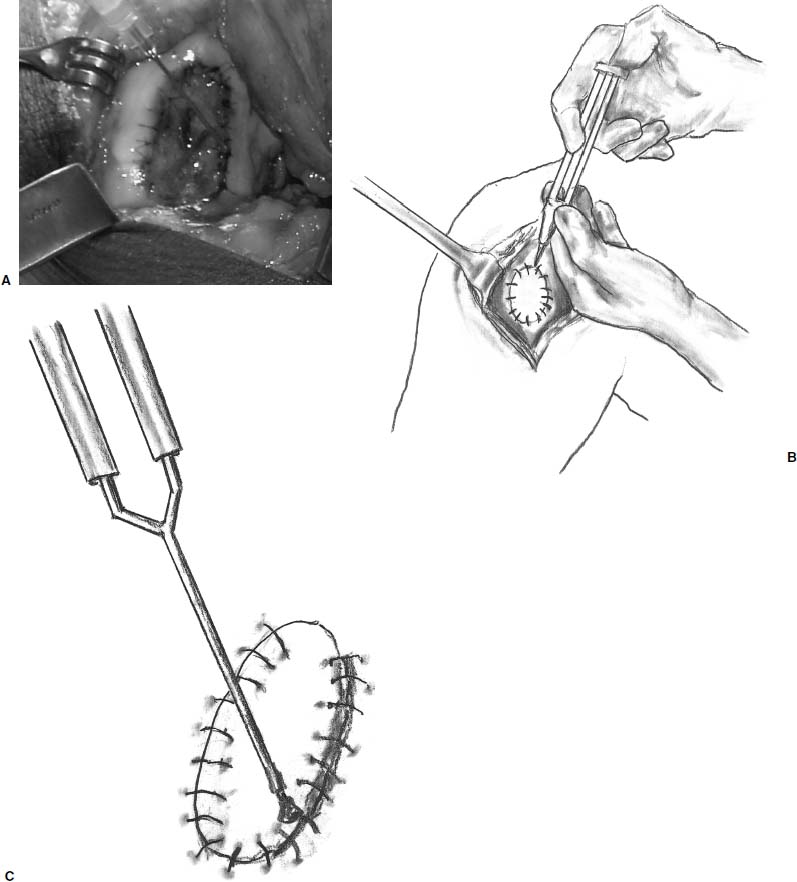
FIGURE 15–14 (A–C) Fibrin glue is then placed over the edges of the graft to obtain a watertight seal.
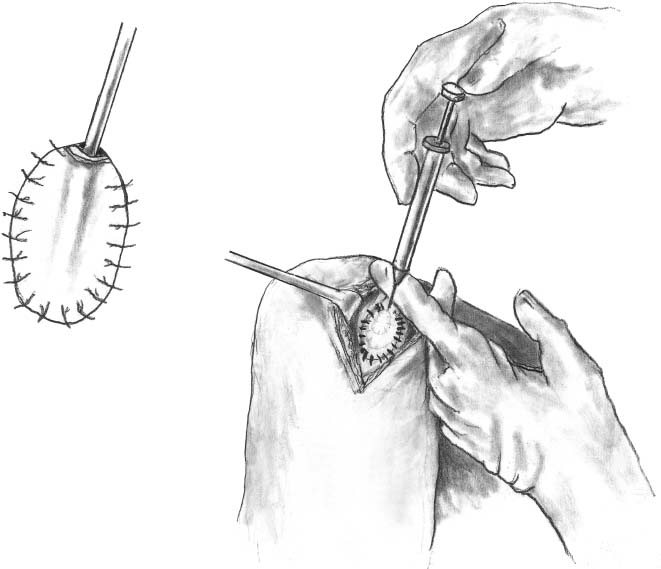
FIGURE 15–15 The cell suspension is then injected into the defect.
In the complex, or salvage cases, when multiple grafts are performed at both articulations, rehabilitation is focused on the region that is deemed to be most involved. Similarly, when concomitant procedures such as ligament reconstruction, osteotomy or meniscal transplant are performed, rehabilitation follows the ACI protocol.
 Complications
Complications
Periosteal graft hypertrophy, graft failure or delamination, and arthrofibrosis are the most common complications following ACI.7 If a patient develops painful catching, new-onset pain, or recurrent effusions in the postoperative period, activity is decreased and an MRI with gadolinium is obtained. Arthroscopy is then performed in select cases. In instances where advanced MRI techniques are not available, diagnostic arthroscopy may be required on a more frequent basis.
In the senior author’s experience, second-look arthroscopy for persistent symptoms was required in 25% of patients.7 Twenty percent were performed due to hypertrophy of the periosteum and 5% performed due to arthrofibrosis following the arthrotomy.
Arthrofibrosis typically develops early (within 3 months) and is treated aggressively with arthroscopic lysis of adhesions, graft assessment, and manipulation under anesthesia. Patients at risk for stiffness were those with a history of keloid formation after prior surgeries, those requiring femoral periosteum for grafts, or those undergoing simultaneous tibial tubercle osteotomy.7 To limit this complication, we currently do not close the knee arthrotomy below the level of the distal pole of the patella. In addition, though it is an off-label use, we use Seprafilm™ (Genzyme Corp., Cambridge, MA) antiadhesive membrane to possibly reduce the incidence of adhesions, as this has been found to reduce adhesions in abdominal surgeries.25
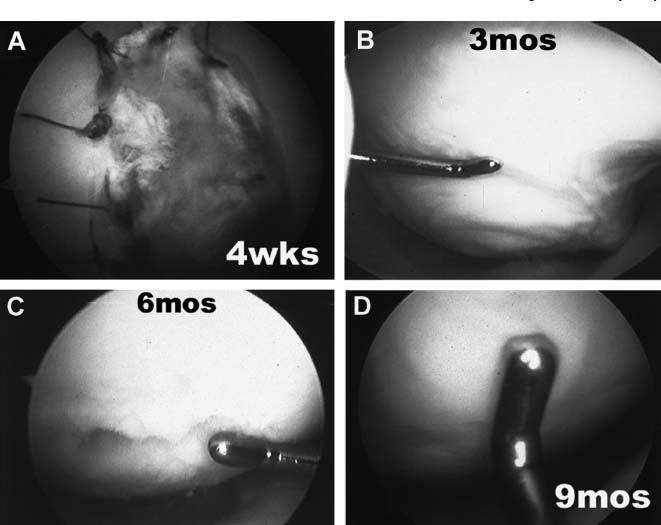
FIGURE 15–16 Arthroscopic photographs of grafts at different stages of healing. (A) At 4 weeks, sutures remain present with complete fill of chondral defect with soft, white tissue. (B) At 3 months the chondral defect is filled with white, soft, jelly-like tissue, which is not firmly attached to the underlying subchondral bone and has a wave-like consistency. (C) At 6 months the graft is putty-like, indentable, and recoils within minutes of being indented. (D) At 9 months the graft is firm and has equal viscoelasticity to the adjacent host cartilage. (Adapted from Minas TM, Peterson L. Autologous chondrocyte transplantation. Oper Tech Sports Med 2000;8:144–157)
Periosteal graft hypertrophy usually presents within the first 3 to 9 postoperative months and presents as a new-onset effusion or painful catching. Gentle arthroscopic debridement of the periosteal overgrowth usually relieves symptoms,6,7 and rarely may be required more than once.
Graft failure has an incidence of 7 to 13% in studies with medium-and short-term results.6,7 Magnetic resonance imaging frequently reveals progressive or persistent subchondral edema under failing graft sites. Graft failure, or delamination, is heralded by a loose flap at the edge of the graft at second-look arthroscopy. Patients are counseled about the possibility of performing marrow stimulating techniques or autologous osteochondral plug transplant to address areas of possible failure prior to arthroscopy. Either of these techniques is utilized when there is a small area of graft delamination (<1.5 cm2). If the area of graft failure is larger, consideration is given to revision ACI, fresh osteochondral allograft, or unicompartmental arthroplasty.
 Results
Results
The first results of ACI were published in 1994 in an initial pilot study from Sweden20; 14 of 16 patients treated with ACI for isolated lesions of the femoral condyle obtained good or excellent results. However, patients treated with lesions of the patella did not fare as well, as only two of seven patients obtained good or excellent results. These results prompted further interest in this technique for full-thickness chondral injuries.
The durability of ACI has been confirmed in a 2- to 9-year follow-up study from Sweden that included the patients treated in the initial pilot study.6 This medium-term retrospective assessment of 101 patients treated with ACI revealed 92% good to excellent results with isolated lesions involving the femoral condyle. The results were further broken down according to diagnosis. Good to excellent results were obtained in 89% of patients treated for osteochondritis dissecans, 75% of patients with concomitant ACL reconstruction, 67% of patients with multiple lesions, and 65% of patients with patellar lesions. The fate of patellar lesions may be improved, however. The authors noted improved results with 11 of 14 good to excellent results with patellar lesions when more attention was given to adequate debridement and patellar realignment when needed.6
The results of the first 169 patients treated with ACI at the Brigham and Women’s Hospital in Boston were released and were encouraging.7 In this short-term follow-up study, 87% of the patients were improved, despite the fact that, “simple,” isolated lesions of the femoral condyle were unusual in this series. Patients were divided into simple, complex, and salvage groups based on the size and number of lesions as well as the presence of early arthritic changes.
Patients were prospectively evaluated and numerous outcome measures used to assess the results. Short Form (SF-36) scores showed clinical improvement in pain relief and were statistically significant in the complex and salvage groups. The simple category did not achieve statistical significance, although 10 of 12 patients were pain free and returned to participation in sports. The Knee Society scores improved in all groups and were statistically significant at 24 months and were maintained at 4 years. Western Ontario McMaster University Osteoarthritis Index scores showed statistical significance at 24 months. Interestingly, the salvage group reported the highest satisfaction of the three groups, with 93% (n = 15 patients at 24 months) reporting they would choose the surgery again.
In general, there was a time-dependent improvement, with maximal improvement occurring by 24 months with femorotibial resurfacing. Patellofemoral resurfacing took longer, 36 months, to reach maximal improvement. Improvements were not as significant when the patellofemoral joint was involved compared with improvements when the tibiofemoral joint was involved. Additionally, when chondral defects were treated with ACI alone, in patients with normal axial alignment, the results seemed superior to those in patients who required osteotomy.
 Future Developments
Future Developments
Improvements for ACI include substitution for periosteum with an off-the-shelf biologic or synthetic membrane, and substances to prevent adhesions such as hyaluronic acid gels or membranes to eliminate periosteal-related problems and arthrofibrosis. Minimally invasive delivery of ACI will only be available after the next generation development of tissue-engineered cartilage, eliminating the need for micro-structuring.
Tissue engineering products that are implanted at a more mature stage of cartilage development along with self-adhering properties to the subchondral bone and adjacent cartilage will dramatically improve clinical outcomes. These will allow arthroscopically performed treatments for small lesions, smaller incisions for large lesions, and a more reproducible histologic repair with a lessened rehabilitation, which translates into less pain, a shorter period of rehabilitation, and a more reproducibly excellent clinical outcome. Tissue engineering will also facilitate a more user-friendly technique that is more widely applicable by the orthopaedist and not as technique sensitive as the present technique of ACI.
 Conclusion
Conclusion
In the past, patients with full-thickness chondral injuries had few treatment options. Today, these injuries may be treated in a variety of ways. Autologous chondrocyte implantation is a technique with encouraging short-term and midterm results that allows predictable results with hyaline-like repair tissue in young, active patients with large (>2 cm2) lesions. Care must be taken to assess comorbid conditions such as axial alignment, ligamentous stability, and meniscal pathology to ensure optimal outcomes. Traditional indications for ACI have been reserved for isolated grade 3 to 4 lesions of the femoral condyle or trochlea with no more than grade 2 chondromalacia of the opposing articular surface.20 However, young, active patients frequently are found to have multiple lesions or early arthritic changes. These injuries can also be treated successfully. Prospective evaluation of patients with validated outcomes analysis suggests that the indications for ACI may be expanded to include complex or salvage patients.7,12 Preoperative counseling is imperative to ensure that patients understand the extensive rehabilitation process that is required as well as the potential complications that may occur. It is hoped that future technologic developments will make ACI a less invasive procedure with more universal application.
REFERENCES
1. Hunter W. Of the structure and diseases of articulating cartilages. Philosophical Trans 1743;470:514
2. Noyes FR, Bassett RW, Noyes FR, et al. Arthroscopy in acute traumatic hemarthrosis of the knee: incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am 1980;62A:687–695
3. Curl W, Krome J, Gordon S. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy 1997;13:456–460
4. Goldberg V. Principles of revision total knee arthroplasty: overview. In: Sim FH, ed. Instructional Course Lectures, vol 50. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2001:357–358
5. Buckwalter JA, Mankin HJ. Articular cartilage: degeneration and osteoarthrosis, repair, regeneration and transplantation. J Bone Joint Surg Am 1997;79A:612–632
6. Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two to nine year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res 2000;374:212–234
7. Minas T. Autologous chondrocyte implantation for focal chondral defects of the knee. Clin Orthop Relat Res 2001;391S:S349-S361
8. Grande DA, Pitman MI, Peterson L. The repair of experimentally produced defects in rabbit cartilage by autologous chondrocyte transplantation. J Orthop Res 1989;7:208–218
9. Brittberg M, Nilsson A, Lindahl A, et al. Rabbit articular cartilage defects treated with autologous cultured chondrocytes. Clin Orthop Relat Res 1996;326:270–283
10. Breinan HA, Minas T, Barone L, et al. Histological evaluation of the course of healing of canine articular cartilage defects treated with cultured autologous chondrocytes. Tissue Eng 1998;4: 101–114
11. Shortkroff S, Barone L, Hsu HP. Healing of chondral and osteochondral defects in a canine model: the role of cultured chondrocytes in regeneration of articular cartilage. Biomaterials 1996;17:147–154
12. Minas T, Peterson L. Autologous chondrocyte transplantation. Op Tech Sports Med 2000;8:144–157
13. Personel communication.
14. Rosenberg T, Paulos L, Parker R, et al. The forty-five degree posterior-anterior flexion weight bearing radiograph of the knee. J Bone Joint Surg Am 1988;70A:1479–1483
15. Winalski C, Minas T. Evaluation of chondral injuries by magnetic resonance imaging: repair assessments. Op Tech Sports Med 2000;8:108–119
16. Outerbridge RE. The etiology of chondromalacia patella. J Bone Joint Surg Br 1961;43B:752–757
17. Recht MP, Kramer J, Marcelis S, et al. Abnormalities of articular cartilage in the knee: analysis of available MR techniques. Radiology 1993;187:473–478
18. Vahlensieck M, Peterfy CG, Wischer T, et al. Indirect MR Arthrography: Optimization and clinical applications. Radiology 1996;200:249–254
19. Peterson L. International experience with autologous chondrocyte transplantation. In: Insall JN, Scott WN, ed. Surgery of the Knee, 3rd ed. Philadelphia: Churchill Livingstone, 2001:341–356
20. Brittberg M, Lindahl A, Nilsson A, et al. Treatment of deep cartilage defects in the knee with autologous chondrocyte implantation. N Engl J Med 1994;331:889–895
21. Riebel GD, Boden SD, Whiteside TE, Hutton WC. The effect of nicotine on incorporation of cancellous bone graft in an animal model. Spine 1995;20:2198–2202
22. Raikin SM, Landsman JC, Alexander VA, Froimson MJ, Plaxton NA. Effect of nicotine on the rate and strength of long bone fracture healing. Clin Orthop Relat Res 1998;353:231–237
23. Buuck DA, Fulkerson JP. Anteromedialization of the tibial tubercle: a 4 to 12 year follow-up. Op Tech Sports Med 2000;8:131–137
24. O’Driscoll SW, Salter R. The induction of neochondrogenesis in free intra-articular periosteal autografts under the influence of continuous passive motion. J Bone Joint Surg Am 1984;66A: 1248–1257
25. Vrijland WW, Tseng LN, Eijkman HJ, et al. Fewer intraperitoneal adhesions with use of hyaluronic acid-carboxymethylcellulose membrane: a randomized clinical trial. Ann Surg 2002;235: 193–199
< div class='tao-gold-member'>