CHAPTER 8
Assessment of Lower Limb Spasticity and Other Consequences of the Upper Motor Neuron Syndrome
Alberto Esquenazi
A number of clinical assessment strategies are routinely used to evaluate the patient with residual consequences of the upper motor neuron syndrome (UMNS). Spasticity is a term that is often used by clinicians, and although frequently used, it can have different meanings in its interpretation and presentation. Spasticity is just one of the many positive signs of the UMNS. The clinical features of the UMNS include negative signs or signs of absence (eg, paresis, loss of dexterity, and selective movement control). Positive signs or signs of presence (eg, different forms of muscle overactivity including the stretch-sensitive and non-stretch-sensitive motor phenomena, such as spastic stretch reflexes, cocontraction, dystonia, enhanced cutaneomotor reflexes, associated reactions, tissue stiffness, and contracture) are a component of the syndrome. Mayer describes in depth these phenomena in Chapter 3 of this book.
The clinical expression of motor dysfunction in the UMNS is strongly influenced by three factors: spasticity and other forms of muscle overactivity, contracture, and impairment of motor control. The time elapsed between the onset of the UMNS and the rehabilitation management of the patient (particularly physical treatment) significantly influences the clinical presentation as rheological changes affecting soft tissue pliability can have a rapid onset. A limb with an upper motor neuron (UMN) dysfunction is vulnerable to loss of range of motion (ROM) and skin, bone, and joint problems, resulting in functional impairment that negatively affects activities of daily living and body image and can produce pain and secondary complications.
Although many scales that intend to assess spasticity concentrate on resistance to passive movement as the main construct, spasticity might also lead to other clinically observable phenomena. Therefore, scales that measure associated clinical phenomena in the context of spasticity, that is, passive ROM (PROM), limb position at rest including postural alignment, tendon reflexes, clonus, spasms, or associated reactions, should be included as part of the assessment. When possible, functional outcomes such as walking should also be included. Evaluation of a patient with a UMN-related movement dysfunction requires a thorough comparison of needs and perceptions reported by the patient and the caregiver with the objective physical examination findings and, when available, movement evaluation in a motion analysis laboratory. There are many assessment techniques used in the routine clinical examination, and for their simplicity and expediency, PROM, motor control, manual muscle strength, Ashworth, and Tardieu scales are the most frequently used in the clinic.
Because clinical scales are based on ratings, they are prone to subjectiveness. Test–retest reliability is a prerequisite for scales that are to be used for evaluation particularly when comparative follow-up is to be performed (1). Intra- and interrater reliabilities are important characteristics that document the potential of a scale to produce stable results within and across clinicians. In essence, it reflects whether a repeated use of the scale can produce stable test results in clinically stable patients.
PROM can be used to determine the available movement for each joint, but it does not provide information on the cause of range limitations if present. Spasticity, muscle overactivity, contracture, and pain can all play a role in limiting PROM. Goniometric measurements can effectively document the PROM and permits comparison to normalcy (Table 8.1).
TABLE 8.1
NORMAL LOWER LIMB JOINT ROM | ||
Ankle | Dorsiflexion/plantarflexion | 0–20/0–50 |
Knee | Extension/flexion | 0/135 |
Hip | Extension/flexion | 0/115 |
ROM, range of motion.
It is imperative to understand the effect of biarticular muscles on joint ROM. Manual muscle testing allows grading of available strength if normal motor control is present; the grading is done using The Medical Research Council 6-point scale, where 5 is a normal rating with the ability to resist significant force and 0 reflects the inability to generate a muscle contraction (Table 8.2).
The clinician needs to recognize that in subjects with UMNS, testing of strength may be affected by impaired motor control, the presence of synergistic patterns, contractures, and communication or cognitive deficits.
The Ashworth Scale allows assessment of muscle tone; the Modified Ashworth uses a 5-point rating scale. The scale has only been validated for the elbow and requires the movement of the joint through its available range in 1 second (2). Ideally, the test should always be done with the subject in the same position and under similar environmental conditions to measure the tone perceived across a joint (3). One disadvantage is that this test does not take into consideration the presence of a contracture or other nonneural components that may limit joint motion (4–6).
The neural component of spastic muscle comes from its stretch reflex activity, whereas the nonneural component comes from rheologic or physical properties intrinsic to muscle and other soft tissues. Many patients with UMNS have a large degree of nonneural resistance the source of which is altered viscoelastic and plastic properties of muscle tissue itself (4,7,8).
When spasticity emerges after a UMN lesion, increased muscle tone may be attributed primarily to its neural component. In time, muscle tone receives an enhanced contribution from its nonneural component when rheologic properties of muscle become stiffer resulting in a contracture. Distinguishing neural from nonneural components of muscle tone has important implications for treatment selection.
TABLE 8.2
THE MEDICAL RESEARCH COUNCIL SCALE FOR STRENGTH | |
0 | No evidence of contraction |
1 | Palpable muscle contraction |
2 | Limb moves with gravity eliminated |
3 | Limb moves against gravity |
4 | Limb moves against resistance |
5 | Normal |
Source: Adapted from Ref. (9). Medical Research Council of the UK. Aids to the Investigation of Peripheral Nerve Injuries. Memorandum No 45. London: Pendragon House; 1976:6–7.
Muscle contracture refers to physical shortening of muscle length, and is often accompanied by shortening of other soft tissues such as fascia, nerves, blood vessels, and skin. A contributing factor to contracture is acute paresis, which develops after UMNS that impairs the normal cycles of shortening and lengthening of agonist and antagonist muscles during everyday voluntary usage. A net balance of contractile forces promotes unidirectional effects on joint position that set the stage for the fixed shortening present in a contracture (7).
The concept of rheologic change after a UMN lesion has literature support from many studies (10). Herman (4) described changes in the rheologic properties of spastic muscles in a study of 220 hemiplegic patients. Patients with contracture often had reduced reflex activity, yet resistance to passive stretch was high because of increased tissue stiffness and contracture.
These studies specify that the understanding of muscle tone should consider the complex interaction between rheologic and spastic properties of muscle because stretch reflexes themselves may be influenced by alterations in the physical properties of muscle. O’Dwyer et al (11) suggested that what appears clinically as poststroke “spasticity” is actually increased muscle stiffness and in some cases contracture. Herbert (7), Gossman et al (12), and Carey and Burghardt (13) suggested that immobility imposed on a patient by the negative signs of UMN can result in soft-tissue contracture.
The Tardieu Scale was developed in the mid-1960s as an ordinal rating assessment tool for the pediatric population; it attempts to assess spasticity by varying the speed of joint motion available from very slow (V1) to as fast as possible (V3). The difference between the parameters permits an estimation of the effect of spasticity (velocity dependence) and takes into consideration the joint ROM limitations imposed by contracture (Figure 8.1) (14).
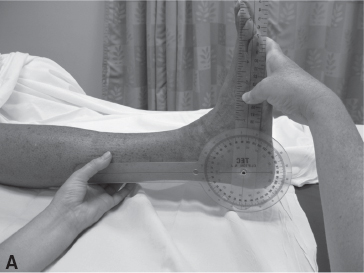
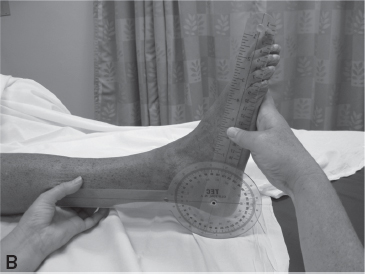
FIGURE 8.1 Low-velocity (A) and high-velocity (B) PROM of the ankle demonstrate the two positions of the ankle during the different measures of the Tardieu Scale with a resulting spasticity angle of –20°.
PROM, passive range of motion.
Voluntary capacity and spastic reactivity are examined and interpreted in light of clinical and functional complaints. Not surprisingly, several outcome measures are used to attempt to evaluate spasticity, pain, function, and disability in patients with UMN problems; other measures include the Penn spasm frequency ordinal rank scale (15) and Biering-Sorensen postural analysis (16). According to Cohen and Marino (17), functional scales estimate the patient’s functional status regardless of the specific underlying impairment. Table 8.3 is a list of the commonly used scales for the assessment of spasticity and associated clinical phenomena.
The selection of outcome measures to assess the functional impact of spasticity is not straightforward, and this is probably a reason why, in most studies of spasticity, disability and function have been evaluated using a heterogeneous collection of outcome measures. Global scales measuring activity limitation such as the Functional Independence Measure and the Barthel Index have not shown to be sensitive enough to record change after the use of interventions such as botulinum neurotoxin (BoNT) for lower limb hypertonicity, and, generally, they have not demonstrated changes directly related to variations of spasticity (18–20).
TABLE 8.3
LIST OF FREQUENTLY USED CLINICAL ASSESSMENT TOOLS AND THE PARAMETERS THEY INTEND TO MEASURE | |
Parameter | Name of Scale |
Resistance to PROM | Ashworth Scale |
Resistance to PROM | Modified Ashworth Scale |
Resistance to PROM | Velocity corrected MAS |
Velocity-dependant resistance to PROM | Tardieu Scale |
Resistance to PROM | VAS for tone |
Resistance to PROM | Tone Assessment Scale |
Hip adductor resistance to PROM | Spasticity score |
Ankle resistance to PROM, tendon jerk, clonus | Total spasticity score |
ROM | ROM with or without goniometer |
ROM | Maximum inter-knee distance |
Resting posture | Ankle position at rest |
Spasm severity | Spasm Severity Scale |
Spasm frequency | Spasm Frequency Scale |
Extensor toe sign | Babinsky response |
Clonus | Clonus score |
MAS, Modified Ashworth Scale; PROM, passive range of motion; ROM, range of motion; VAS, Visual Analog Scale.
Richardson et al (21) and Johnson et al (22) have demonstrated functional improvement in mobility using the Rivermead Motor Assessment Scale. Likewise, one placebo-controlled, randomized control trial using BoNT and electrical muscle stimulation to assist gait retraining for spastic foot drop and one prospective case series using BoNT to treat stiff knee syndrome after stroke have been unable to demonstrate changes in health-related quality of life using the 36-Item Short-Form or in social participation using the SATISPART-Stroke (22–25).
The Goal Attainment Scale can be used to grade the achievement of outcomes following treatment with BoNT, with a focus on improvements of function and participation, which are relevant to the patient or his or her caregivers (26).
More recent publications have reported a link of functional outcomes based on the Physician Global Assessment Scale Score for the upper limb to the Ashworth-based clinical measure of tone (27). Unfortunately, none of them has provided a meaningful correlation with lower limb functional performance of common activities, such as walking velocity, and none has precisely identified the source of the problem impairing function. These deficiencies in part may be due to the fact that most indices quantifying function are ordinal; they merely rank persons with little input into how people function or how they are rehabilitated. For example, a total Functional Independence Measure score is a composite value of its multiple components, and without an item-by-item analysis, the ability to discern where the deficits are and how to intervene to attempt a solution is not possible. Based on our clinical experience, assessment methods based on a functional perspective or functional impairment such as those described later in this chapter may be more helpful in this regard.
Each year in the United States, approximately 700,000 people are affected by a stroke, and many more sustain a traumatic brain injury (TBI). These are the two prevalent forms of acquired brain injury producing an UMNS in the adult population. Acquired brain injury affects a person’s cognitive, language, perceptual, sensory, and motor function (28). There are more than 1,700,000 persons in the United States with residual functional impairment after stroke and TBI (29,30).
Dysfunction in the corticospinal tract and other descending pathways involved in voluntary motor activity is seen frequently. The immediate consequences may include paralysis and joint immobilization, with symptoms such as weakness, stiffness or rigidity, decreased manual dexterity, slowed movement, and fatigue. The loss of inhibitory impulses from higher centers in the central nervous system allows excessive muscle activity. Symptoms that may accompany lower limb spasticity include involuntary muscle spasms, clonus, scissoring of legs, abnormal limb postures, and joint contractures. Recovery is a long process that continues beyond the hospital stay and into the home setting. The rehabilitation process is guided by clinical assessment of motor and cognitive abilities. Accurate assessment of the motor abilities is important in selecting the different treatment interventions available to a patient. Ideally, a team approach to the evaluation of this patient population, their residual deficits, and the resulting limb postures is necessary, as the disabling forms of muscle overactivity affecting patients with UMNS are a widespread problem with functional implications for which a variety of interventions exist to address them. Spasticity is not always harmful; some patients rely on their spasticity for functional activities such as walking or standing. For others, however, spasticity can be painful and distressing (31).
More than half a century ago, Nikolai A. Bernstein suggested that a basic problem of motor control relates to overcoming redundant degrees of freedom in our multijointed skeletal system that allow us to interact with the three-dimensional world in which we live. Commonly, there are multiple agonist and antagonist muscles called upon for virtually any movement direction. To match a required joint torque even across a single joint, the questions regarding which muscles should be activated and how much force will it generate are likely to have variable answers without an exclusive solution. For a given circumstance, for example, there may be a unique solution in that equinovarus deformity may be solely attributable to an overactive tibialis anterior in one patient, whereas in another, it may be the result of an overactive tibialis posterior (32). Simply put, identifying muscles that produce deforming maladaptive joint movements and postures statically and dynamically is an important endeavor in aiding clinical interpretation of lower limb dysfunction and its impact on gait, and in rationalizing available treatment interventions and assessing the result of such intervention.
Clinical evaluation is useful to the analysis of movement dysfunction, but gait and movement laboratory evaluation using dynamic electromyography (EMG) is often necessary to confidently identify the particular contributions of offending muscles (Figure 8.2).
The therapies for management of UMNS-related lower limb spasticity and muscle overactivity include exercises for stretching, strengthening, and coordination. Physical modalities such as casting and splinting, the use of focal neurolitic blockades, systemic medications and intrathecal drugs, neuro-orthopedic and neurosurgical interventions, and other forms of therapies are also used (Figure 8.3).
Problems of movement control and limb deformities are common consequences of UMNS. Dynamic EMG, gait analysis, and diagnostic nerve blocks frequently provide the necessary detailed information about specific muscle groups that will guide decision making for treatment. Ideally, all treatment interventions must be patient-focused based on a multidisciplinary assessment that will result in targeted interventions to achieve patient-specific goals. The correct selection of target muscles that contribute to any one pattern of dysfunction may serve as a rational basis for interventions that focus on specific muscles for therapy (focal intervention). Sheean (33) and Mayer and Esquenazi (34) have proposed useful classifications of function for this patient population (Table 8.4). Functional goals may be classified as active, passive, or symptomatic in nature and overlap nicely with the International Classification of Functioning, Disability, and Health (ICF) framework, which is based on the biopsychosocial model covering function and disability with its components—body structures, body functions, activities and participation—as well as personal and environmental factors (35).
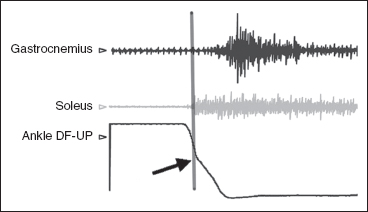
FIGURE 8.2 Ankle Ashworth test with dynamic EMG of ankle plantar flexors recorded with multichannel Motion Lab System and three-dimensional movement tracing of ankle (black line) recorded with CODA CX1 (Charnwood Dynamics). Note the increased activation of the soleus and then gastrocnemius during the passive dorsiflexion phase. Note the gradual slope of the ankle motion curve (the catch) caused by soleus overactivity as marked by the vertical line.
EMG, electromyography.
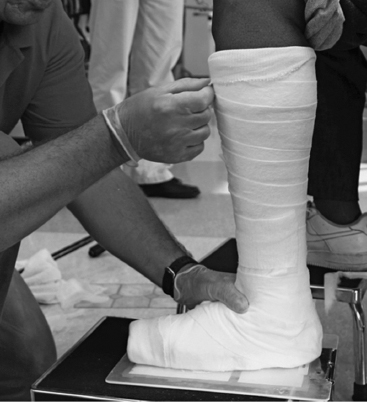
FIGURE 8.3 Patient with left ankle serial casting used to stretch a ROM limitation and reduce the point at which a spastic reaction occurs. The cast will be replaced at 3-day intervals and applied for a total of 9 to 12 days.
ROM, range of motion.
TABLE 8.4
CLASSIFICATION OF UMNS-RELATED PROBLEMS FOR TREATMENT GOAL DEVELOPMENT |
Type I: Symptomatic |
UMNS, upper motor neuron syndrome.
A symptomatic goal (type I) refers to clonus, flexor, or extensor spasms and pain among others as some of the targeted goals. However, it is also important to consider the impact on function. Application of lower limb orthoses, transfers and standing balance, perineal hygiene or catheterization, facilitation of therapy, and decrease of burden of care are examples of passive function (type II), where a carer carries out a task or where the individual tends to the affected limb with the unaffected limb. Options for assessment of passive function include verbal or visual analogue ratings of “ease of care,” “timed care tasks” (eg, time taken for dressing), and formal scales that measure dependency or carer burden. Digital photography before and after treatment can provide a useful record of skin breakdown caused by pressure or difficulty with skin hygiene. Some studies have shown improvement in passive functions such as ease of hygiene and dressing after treatment with BoNT or ability to tolerate orthoses (36–38).
Mobility, transfers, activities of daily living, and sexuality are examples of active function (type III), where the individual carries out a functional task (32).
A number of standardized scales can be used to compare outcomes between individuals and groups. The Leeds Adult Spasticity Impact Scale (39) is an example. The reality is that most global scales of independence in activities of daily living are rarely sensitive to focal interventions for lower limb spasticity. Based on this, the goals for treatment will determine the appropriate scale.
When assessing a patient for development of a treatment plan, the clinician should consider the following:
• Is the presenting problem preventing function or affecting independence?
• Is there limb pain or other symptoms that may impact quality of life?
• The treatment options previously employed and what were the results of those interventions (40)?
• Overall health status of the patient and the expected therapeutic goals.
• The severity and scope of the problem, that is, local versus regional versus generalized problems (41).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






