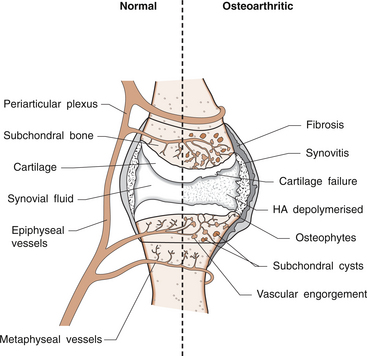
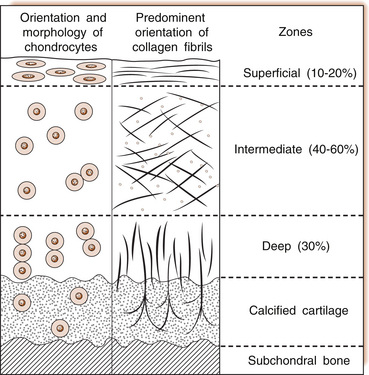
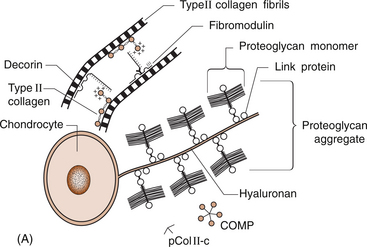
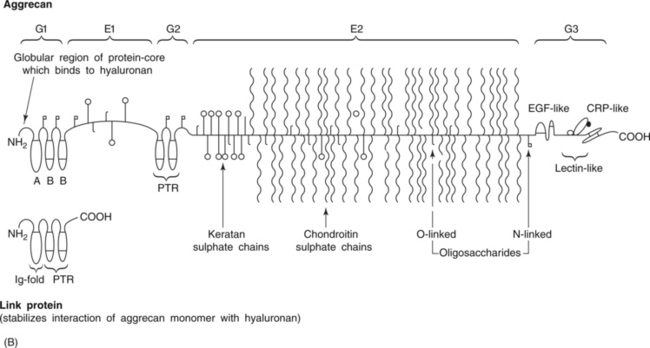
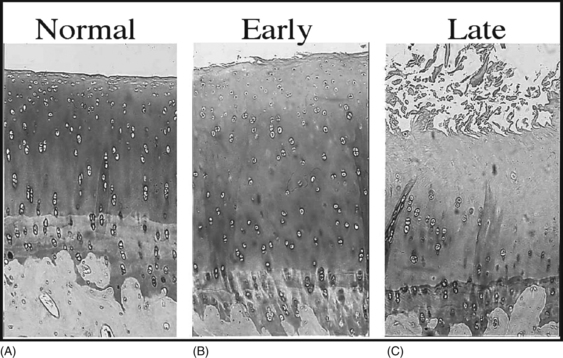
6 Lyn March and Chris Little The knee is a synovial joint with a surrounding capsule, an internal synovial lining that produces the lubricant joint fluid, internal menisci and stabilizing ligaments, and articular cartilage covering the surface of the bones. In the pathogenesis of osteoarthritis, changes occur in all structures including the synovial lining layer, the synovial fluid, the articular cartilage and the subchondral bone (Fig. 6.1). It is felt that the earliest and most significant changes occur in the articular cartilage and this chapter will focus on these. Articular cartilage is a specialized form of connective tissue that covers and protects the ends of the bones in synovial joints. For the knee joint this makes up the smooth surfaces covering the femoral and tibial condyles and the under surface of the patella. The surface is smooth and slippery with an extraordinarily low coefficient of friction, while the deeper layer merges with a calcified layer (the tidemark) that interlocks with the subchondral bone (Fig. 6.2). During skeletal development, the articular cartilage forms from very densely packed mesenchymal cells that differentiate into chondrocytes, which proliferate rapidly and synthesize the large amounts of extracellular matrix. The extracellular matrix is made up predominantly of water (up to 80%), collagen and proteoglycans (discussed below), which are produced and maintained by the relatively sparse cells, the chondrocytes. It is the combination of collagen, proteoglycan and water that gives articular cartilage its unique properties. The collagen forms a network of fibrils that gives the overall framework and shape of the cartilage and provides pockets or compartments that are filled with the water-binding proteoglycan complexes that regulate the compressibility. The ability of articular cartilage to resist compressive deformation is largely due to the entrapment of high concentrations of the polyanionic large proteoglycan aggrecan within the collagen fibrillar network. The osmotic pressure provided by the glycosaminoglycan (GAG) chains on the aggregated aggrecan molecules is resisted by and constrained within the insoluble collagenous meshwork. Degradation of cartilage is a central pathological feature of arthropathies such as osteoarthritis and rheumatoid arthritis, and involves proteolytic cleavage of both its major structural elements, namely aggrecan and type II collagen. Proteolysis and subsequent loss of the GAG-rich region of aggrecan from cartilage is an early event in cartilage degeneration, while significant catabolism of the collagen fibrillar structure occurs later and may represent the point of irreversible cartilage damage. Chondrocytes vary in shape, size and number of cells per area depending on their different anatomical locations, even within different regions of the normal knee joint. Close to the surface they are flatter, smaller and generally have a greater density than the cells deeper in the matrix. Each chondrocyte sits within a lacuna (space). Collagen fibres come right up to the edge of the lacunae, which are filled with fine fibrillar material. Unlike the osteocyte in bone, their cytoplasmic processes do not make contact with processes of other chondrocytes i.e. they have little or no cell-cell contact. They have low numbers of mitochondria, which reflects their low oxygen consumption rates. In adult cartilage, the rate of cell division is very low but division does occur in response to injury or disease. Chondrocytes, particularly in the deeper uncalcified zone, have prominent endoplasmic reticulum and Golgi apparatus responsible for protein synthesis and sulphation of the mucopolysaccharides that form the proteoglycan side-chains. Lipid occurs intracellularly and as a diffuse layer around the cells, and probably contributes to cartilage lubrication. When the delicate balance of cartilage matrix synthesis and destruction is upset, the net result is articular cartilage loss and the degenerative process that ensues is osteoarthritis. Despite significant advances in our understanding of cartilage structure, there is still a lot to learn about the mechanisms of cartilage destruction. While it is felt that the initial pathology in osteoarthritis involves the articular cartilage, it is evident from very early stages that there are changes also in the synovial lining, the synovial fluid and the underlying subchondral bone. Pathology occurs in all joint tissues in OA, including bones, menisci, ligaments and synovium but the central feature is progressive degeneration of the articular cartilage (Fig. 6.4). Articular cartilage has a very poor reparative capacity, and ultimately it is the breakdown and erosion of this tissue that signifies end-stage arthritis necessitating joint replacement surgery. Cartilage is aneural, avascular, sparsely populated with cells (chondrocytes) and predominantly comprised of extracellular matrix (Fig. 6.4A). The major matrix proteins are type II collagen which forms a structural scaffold, and aggrecan (the aggregated proteoglycans) which is substituted with negatively charged glycosaminoglycans. The swelling pressure of the aggrecan is balanced by the tension in the collagen network, and together they endow cartilage with its resilience under compression. Progressive breakdown of cartilage in OA involves proteolysis of both aggrecan and type II collagen. Aggrecan breakdown precedes and is independent of collagen degradation, and both in vitro and in vivo is due to the action of members of the A Disintegrin And Metalloproteinase with ThromboSpondin motif (ADAMTS) family of enzymes. In particular ADAMTS-4 and -5 appear to be the physiologically important ‘aggrecanases’. Although cartilage has a limited ability to repair, aggrecan can be replenished by the chondrocytes and the mechanical properties of the tissue restored if the destructive insult is removed prior to disruption of the collagen network. Once significant disruption of the collagen network occurs (Fig. 6.4C), cartilage damage is irreparable with further collagen breakdown resulting in progressive cartilage loss. MMPs are secreted as zymogens and must be activated extracellularly where their activity is then controlled by tissue inhibitors of metalloproteinases (TIMPs). Chondrocyte mRNA levels for numerous MMPs including MMP-2, -9 and -13 are significantly increased in OA cartilage in both animals and humans. While OA is often considered a ‘non-inflammatory’ arthropathy, chondrocyte-derived cytokines such as IL-1 and TNF are known to play a direct role in human cartilage degradation by MMPs. However, the precise regulatory pathway whereby chondrocyte MMP expression is regulated, and therefore the identification of potential therapeutic targets, is still the subject of intensive investigation. As discussed in Chapter 1, the normal synovium consists of two layers: the lining layer of cells, known as the intima, and the remaining subsynovial tissue or subintima. The normal intima is formed by an interlacing layer, one to three cells (synoviocytes) deep, that merges with the underlying connective tissue. There is no basement membrane between. The lining is generally smooth with few folds and the subintima has connective tissue cells (fibroblasts) only and relatively few blood vessels and endothelial cells. Normal synovial fluid is clear, pale yellow in colour and very sticky or viscous (see Ch. 1). It is a dialysate of plasma combined with hyaluronic acid that is produced by the synovial cells. The molecular weight of HA determines the elasticity and viscosity of the fluid. A normal knee joint contains 0.5–1.5 mL of fluid, which coats the surface of the articular cartilage, providing lubrication for movement, a vehicle for flow of nutrients from the fluid into the articular cartilage matrix and a shock-absorbing cushion on weight bearing. In OA there is often an increased volume of fluid but the hyaluronic acid content and thus the viscosity and other mechanical properties of the fluid are reduced. The fluid is still usually clear and non-inflammatory in OA but if cartilage fragments and/or low-grade inflammation are present, it will have a more turbid appearance.
ARTICULAR CARTILAGE IN HEALTH AND DISEASE
Anatomy
Articular cartilage
The chondrocyte
Biochemistry
Pathophysiology of osteoarthritis
Articular cartilage
What mechanisms are important in cartilage breakdown?
Activation of MMPs in cartilage
Synovium
Synovial fluid
ARTICULAR CARTILAGE IN HEALTH AND DISEASE