, James B. Galloway2 and David L. Scott2
(1)
Molecular and Cellular Biology of Inflammation, King’s College London, London, UK
(2)
Rheumatology, King’s College Hospital, London, UK
Abstract
There are currently five TNF-α inhibitors available to treat inflammatory arthropathies. These can be subdivided into first generation agents (comprising etanercept, infliximab and adalimumab) and second generation agents (comprising certolizumab and golimumab). In RA all TNF-α inhibitors are nationally approved for use in routine clinical care. There is no evidence that one of these agents is superior to another and practical and financial issues determine which is chosen. In other forms of inflammatory arthritis, in particular in AS, there is evidence that some biologics may be less effective than others. Overall, there is strong clinical trial evidence for their efficacy in inflammatory arthritis. Although relatively safe, TNF-α inhibitors have a broad range of potential adverse effects. The dominant risk is an increased infection risk, particularly that of latent tuberculosis reactivation. This chapter will provide an overview of the available TNF-α inhibitors, their mechanisms of action, the evidence base for their efficacy and the potential adverse events associated with them.
Keywords
TNF-αEtanerceptInfliximabAdalimumabCertolizumabGolimumabSide-EffectsTuberculosisBackground
Early research in the 1980s and 1990s highlighted the importance of cytokines in inflammatory arthritis. It showed many different pro- and anti-inflammatory cytokines were over-expressed in the rheumatoid synovium. Although there was debate about whether IL-1 was the major driver in inflammatory arthritis, research suggested that TNF-α might have a central role. The proof of principle for inhibiting this cytokine came from an open label clinical study in which patients with RA received a single infusion of a TNF-α inhibitor. This showed a rapid response, including an early fall in CRP levels [1]. However, the anti-inflammatory effect lasted only 6–12 weeks. It was followed by a return of active disease. As a result patients were retreated with further infusions and they showed responses of a similar magnitude and duration. The scene was set for a major clinical development programme. One concern was that the TNF-α inhibitor, which included mouse protein, might lose efficacy due to immunogenicity. Up to 60 % of Infliximab treated patients develop anti-drug antibodies [2]. This was solved by giving methotrexate as an additional treatment. This has become a standard approach with these biologics.
There are currently five TNF-α inhibitors available to treat inflammatory arthropathies (Table 8.1). These can be subdivided into first generation agents (comprising etanercept, infliximab and adalimumab) and second generation agents (comprising certolizumab and golimumab). In RA all TNF-α inhibitors are nationally approved for use in routine clinical care. There is no evidence that one of these agents is superior to another, and practical and financial issues determine which is chosen. In other forms of inflammatory arthritis, in particular in AS, there is evidence that some biologics may be less effective than others.
Table 8.1
Currently available TNF-α inhibitors for the treatment of inflammatory arthritis
TNF-α inhibitor | Site of action | Dosing schedule | Methotrexate |
|---|---|---|---|
Infliximab | Binds soluble and transmembrane TNF-α and inhibits binding of TNF-α to TNF receptors | IV administration every 4–8 weeks | Essential to co-prescribe |
Etanercept | Soluble TNF-receptor fusion protein that binds TNF-α and TNF-β preventing them from interacting with their receptors | Subcutaneous once or twice weekly | Optional to co-prescribe |
Adalimumab | Binds soluble and transmembrane TNF-α and inhibits binding of TNF-α to TNF receptors | Subcutaneous fortnightly | Optional to co-prescribe |
Certolizumab | Binds soluble and transmembrane TNF-α and inhibits binding of TNF-α to TNF receptors | Subcutaneous fortnightly | Optional to co-prescribe |
Golimumab | Binds soluble and transmembrane TNF-α and inhibits binding of TNF-α to TNF receptors | Subcutaneously monthly | Optional to co-prescribe |
Etanercept
Etanercept is a soluble TNF-receptor fusion protein. It comprises two dimers. Each dimer has an extracellular, ligand-binding portion of the higher-affinity type 2 TNF receptor (p75) which is linked to the Fc portion of human IgG1. This fusion protein binds to both TNF-α and TNF-β and prevents them from interacting with their receptors. It is given by subcutaneous administration at a dose of 25 mg twice a week or 50 mg once a week. This dosing reflects its half-life of approximately 4 days. Interestingly etanercept has never received a license for inflammatory bowel disease because of lack of efficacy in clinical trials [3].
Infliximab
Infliximab is a chimeric IgG1 anti–TNF-α antibody in which the antigen-binding region is derived from a mouse antibody and the constant region from a human antibody. It binds to soluble and membrane bound TNF-α with high affinity. This binding impairs the binding of TNF-α to its receptor. Infliximab also kills cells that express TNF-α through antibody- dependent and complement-dependent cytotoxicity. There are considerable differences between patients in the pharmacokinetics of infliximab. The intravenous dosing method of infliximab results in much greater initial peak concentrations along with higher peak to trough ratios when compared to other anti-TNF agents [4]. Trough concentrations, seen 8 weeks after intravenous administration of 3 mg/kg of infliximab vary enormously between patients. Shortening the interval between doses may be more effective in increasing the trough levels than increasing the dose. In RA patients the dose given is 3 mg/kg every 8 weeks. In PsA and AS the dose is 5 mg/kg every 8 weeks. Some patients have higher doses, and in certain circumstances the intervals between doses can be reduced.
Adalimumab
This is a recombinant human IgG1 monoclonal antibody. It binds human TNF-α with a high affinity, and as a consequence inhibits this cytokine binding to its receptors. It also lyses cells that express TNF-a on their surface. It is given by subcutaneous injection and is then absorbed slowly. Peak concentrations are achieved after 120 h. Although there are wide variations between patients, it is given fortnightly at a dose of 40 mg.
Certolizumab
Certolizumab pegol is a recombinant humanised Fab fragment (which is the antigen-binding domain) of a TNF-α antibody, which has been coupled to polyethylene glycol (PEG). This coupling prolongs its plasma half-life to approximately 2 weeks. It binds and neutralises membrane-bound and soluble human TNF-α. It is the only TNF-α inhibitor without an Fc region. It is given by subcutaneous injection with an initial “loading dose” of 400 mg every 2 weeks for 6 weeks, followed by 200 mg once fortnightly.
Golimumab
Golimumab is a human IgG1 monoclonal antibody against TNF-α, which is produced in a transgenic mouse. It targets and neutralises both soluble and membrane bound TNF-α. It has a variable half-life of between 7 and 20 days. It is given as a monthly subcutaneous injection at dose of 50 mg, which can be increased to 100 mg a month if the patient’s body-weight is more than 100 kg.
Indications
Rheumatoid Arthritis
TNF-α inhibitors should be initiated when patients continue to have active RA (defined as a DAS28 score of >5.1 on two occasions at least 1 month apart) after an adequate trial of effective DMARDs (defined as 6 months of treatment with at least 2 DMARDs, one of which must be methotrexate). Unless contra-indicated they are given with methotrexate, which increases efficacy and helps to prevent the formation of anti-biologic antibodies. Their use as the first line for the treatment of RA should at present be limited to research studies.
Psoriatic Arthritis
As with RA, TNF-α inhibitors in PsA are restricted to patients who continue to have active disease despite DMARD therapy. Current national guidelines recommend their use in patients with active PsA (defined as a tender and swollen joint count of at least 3) despite treatment with two DMARDs (given for 6 months, one of which is methotrexate).
Ankylosing Spondylitis
TNF-α inhibitors are of proven efficacy in AS. They are used in patients who have not responded to treatment with NSAIDs. NSAID failure is defined as an inability of NSAID drugs taken sequentially at maximum tolerated doses for 4 weeks to control symptoms. Patients require active disease with a BASDAI score of at least four units and a spinal pain VAS of at least 4 cm.
Clinical Effects
Rheumatoid Arthritis
TNF-α inhibitors given in adequate doses produce major improvements in symptoms, signs, and laboratory measures. This improvement occurs within 12 weeks of starting treatment. Switching from one TNF-α inhibitor to another is well documented, with supporting evidence from clinical trials.
Individually important responses should occur within 8–12 weeks. Provided there is some evidence of benefit, treatment should be continued. If patients show no response therapy should be altered. In patients with an incomplete response, there is some evidence that increasing the dose or reducing dosing intervals may provide additional benefit, as may the addition or substitution of other DMARDs.
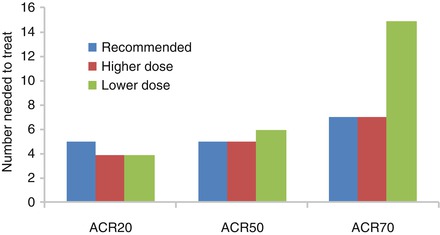
A systematic review of the efficacy of TNF-α inhibitors in RA showed the number needed to treat was 5 to obtain an ACR20 response and 7 to obtain an ACR70 response (Fig. 8.1) [5]. Clinical features like joint counts, disability and joint damage were all improved or progressed less with TNF-α inhibitor use. There was no reason to prefer one TNF-α inhibitor over another. There was limited evidence that giving lower or higher doses changes the effect of biologics, though lower doses reduced the chance of developing an ACR70 response.