Anterior Spinal Fusion for Thoracolumbar Idiopathic Scoliosis
Franklin Keith Gettys
Daniel J. Sucato
INDICATIONS/CONTRAINDICATIONS
The anterior approach for thoracolumbar/lumbar curves in adolescent idiopathic scoliosis (AIS) is extremely effective and a safe method to treat those curve patterns that continue to show progression to a level associated with progression in adulthood despite conservative measures. The indications include thoracolumbar/lumbar curves (Lenke 5C) greater than 40 to 45 degrees in the adolescent age group and may be applied to patients who are in the juvenile age group. Contraindications to the anterior approach for these thoracolumbar/lumbar curves are relatively few and may include those patients who have had previous intra-abdominal surgery since significant scar formation would be present, making access to the spine more challenging, or a patient who has an associated structural thoracic curve (1, 2).
PREOPERATIVE PLANNING
Patients who plan to undergo an anterior spinal fusion and instrumentation (ASFI) for thoracolum-bar/lumbar curves should be evaluated both clinically and radiographically. The clinical examination should focus on the patient’s trunk balance, spinal deformity, waistline asymmetry, and neurologic status. Assessment of trunk balance should determine whether a trunk imbalance is present and its direction. If a thoracic curve appears to have some structural characteristics clinically, then an ASF of a thoracolumbar/lumbar curve would accentuate the prominence and result in a poor cosmetic result (2). The typical patient with a thoracolumbar/lumbar curve who would require surgery will have a trunk shift to the convexity of that curve (usually left side), which can be quantified by measuring the deviation from a plumb bob to the center of the sacrum. This is also associated with significant waistline asymmetry. Neurologic examination is always important in these patients to ensure that there is no indication to obtain an MRI to assess for neural axis abnormalities (3). The abdominal reflexes are especially important in the assessment of the patient with presumed idiopathic scoliosis and should be symmetric (bilaterally absent or present).
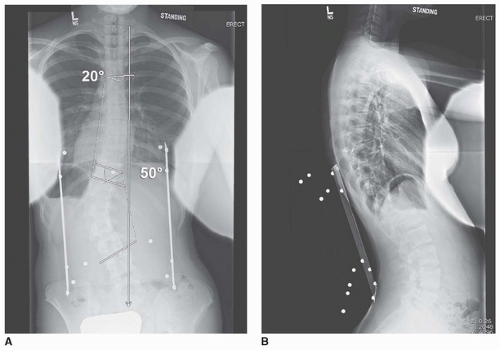
Radiographic assessment should rely on the periapical (PA) as well as the lateral radiograph. The PA radiograph is used to measure the coronal measurements of the upper thoracic, main thoracic, and thoracolumbar/lumbar curves. It should be used to assess the apical vertebral translation to ensure that this is truly a structural thoracolumbar/lumbar curve without a structural thoracic curve. The skeletal maturity can be assessed by evaluating the status of the triradiate cartilage and the Risser sign. The lateral radiographs should be used to measure the amount of lumbar lordosis. Junctional kyphosis between the main thoracic and thoracolumbar/lumbar curve would indicate structurality of both curves and preclude isolated fusion and instrumentation of the thoracolumbar/lumbar curve. The supine bend radiographs can be used to assess the flexibility of the curve. This is important in preoperative planning and may dictate the extent of the discectomy and whether resection of the posterior annulus and the posterior longitudinal ligament (PLL) is necessary to obtain satisfactory correction.
The PA radiograph is the main imaging study to plan surgical levels (Fig. 37-1). The most accepted fusion level planning is to include all vertebrae within the thoracolumbar/lumbar curve (inclusion
from proximal to distal end vertebrae). The most common thoracolumbar/lumbar curve is measured from T11 to L3, and they are the most common fusion levels. Supine best-bend radiographs do not generally assist in choosing fusion levels but are utilized to ensure that the thoracic curve is not structural.
from proximal to distal end vertebrae). The most common thoracolumbar/lumbar curve is measured from T11 to L3, and they are the most common fusion levels. Supine best-bend radiographs do not generally assist in choosing fusion levels but are utilized to ensure that the thoracic curve is not structural.
Hall et al. have popularized the concept of a short anterior fusion. The criteria for this technique included curves less than 60 degrees, which were relatively flexible, and fusion levels were based upon whether the apex was at the disc or at a vertebral body. If it were at a vertebral body, then fusion one segment above and one segment below this apical vertebral body would yield a satisfactory correction. When the apical segment is a disc, then fusion and instrumentation would occur two segments above and two segments below this apical disc. It is important to understand that overcorrection of the curve would be necessary to achieve good results using this strategy (4).
SURGICAL PROCEDURE
The anterior approach for instrumentation and fusion of a thoracolumbar/lumbar curve is performed with the patient in the lateral decubitus position. The convexity of the curve is positioned up so that the access is achieved onto the convexity of the spine. Generally, the rib just proximal to the proximal instrumented vertebra is utilized. For example, fusion from T11 to L3 would require an incision over the 10th rib and gaining access to the chest and abdomen through the 10th rib. After the patient is positioned in the lateral decubitus position and an axillary roll is placed, the patient is secured with a beanbag ± body positioners and the table can be flexed to provide greater access to the spine. The patient’s flank and back are then prepped in the normal sterile fashion. The spine is usually able to be palpated and the umbilicus can also be visualized, to maintain anatomic perspective.
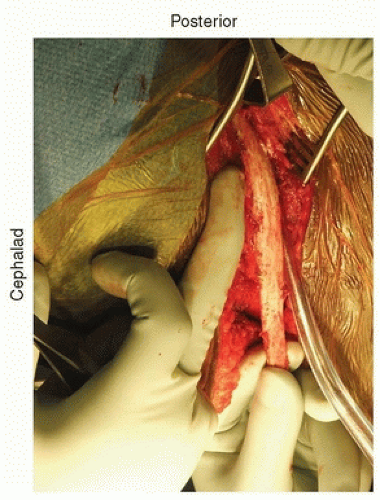
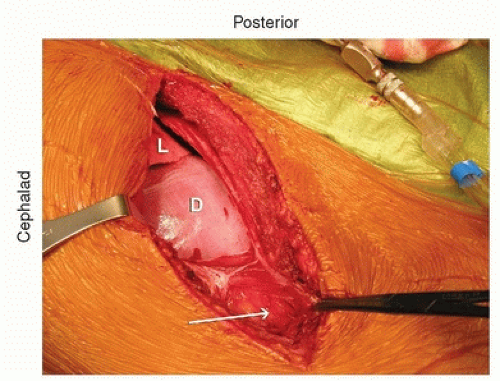
The incision is directly over the rib and extends in line with the rib. It is then extended down just lateral to the midline and extends depending on the length of fusion. Posteriorly, it is extended back close to where the rib inserts at the transverse process. If the incision is carried through the various muscle layers down to the rib periosteum, which is incised, subperiosteal dissection around the rib is then performed (Fig. 37-2). Usually, the 10th rib is harvested, and direct access into the chest can then be achieved through this incision. The rib is harvested then by cutting it as posteriorly as possible with the rib cutter. It is then disarticulated from the costochondral junction. The cartilage is then incised in a longitudinal fashion, and this gets you right down to the retroperitoneum. The retroperitoneal space can be identified by the retroperitoneal fat (Fig. 37-3). The peritoneum is then bluntly dissected off the abdominal wall and the diaphragm (Fig. 37-4). As one does this, the diaphragm is incised, leaving a 2-cm cuff of diaphragm distally (Fig. 37-5). Marking stitches should be placed to allow for reapproximation of the diaphragm at the end of the procedure.
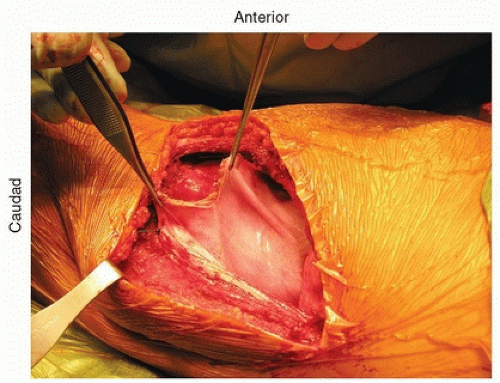
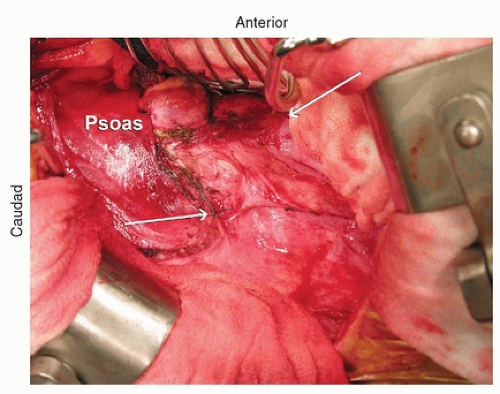
As one dissects down toward the spine, the parietal pleura is identified. The peritoneum continues to be dissected off the abdominal wall, and the psoas then comes into view. The psoas inserts at L1
and should be partially dissected off the spine and retracted posteriorly to provide good access to the spine (Fig. 37-6). Once the diaphragm is dissected down to the parietal pleura, the parietal pleura is incised and the segmental vessels can be easily identified (Fig. 37-7).
and should be partially dissected off the spine and retracted posteriorly to provide good access to the spine (Fig. 37-6). Once the diaphragm is dissected down to the parietal pleura, the parietal pleura is incised and the segmental vessels can be easily identified (Fig. 37-7).
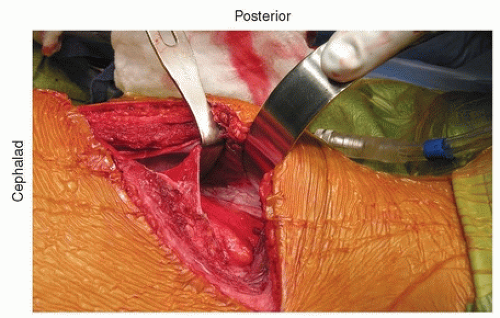 FIGURE 37-4 The peritoneal contents are bluntly dissected off the abdominal wall. The diaphragm is being retracted proximally/posteriorly. |
At this point, a chest spreader and/or Balfour retractor is placed to give good visualization within the chest and abdomen, respectively (Fig. 37-8).
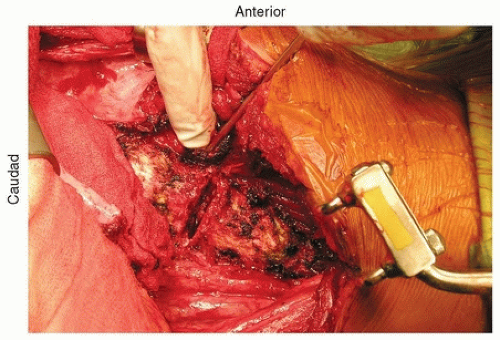
The segmental vessels should be ligated. It is important to avoid hypotensive anesthesia during these anterior surgical cases since the segmental blood vessels will be ligated for access. Segmental vessels should be isolated using a right angle hemostat, which can then be used to pass a nylon suture, which are then tied and the vessel is incised (Fig. 37-7). The cut segmental vessels are then retracted anteriorly and posteriorly using blunt dissection and sharply freeing any soft-tissue attachments to the vessels.
Complete removal of the disc material is the most important aspect of the procedure to ensure a solid arthrodesis. This requires complete exposure of the disc to its posterior edge and all the way anterior and around to the contralateral side of the spine. One should be able to palpate to the opposite annulus.
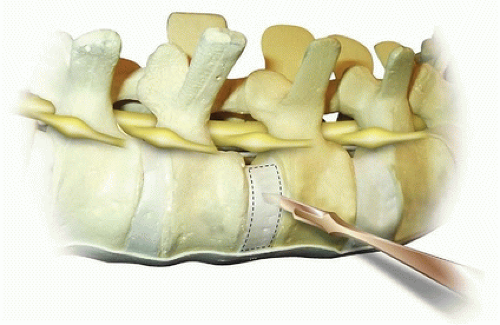
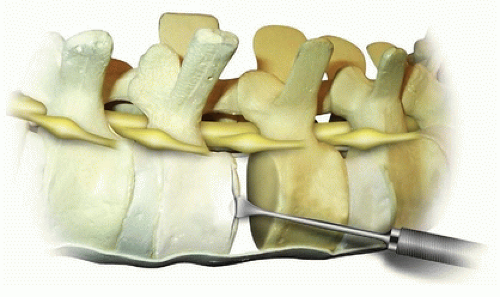
The disc can be incised with a sharp scalpel blade from anterior to posterior in a box-type cut (Fig. 37-9). This is then incised midway so that the annulus is easier to remove with the rongeur. Incision of the adjacent endplate should be performed at the junction between the vertebral body and the endplate. The periosteum is incised as far circumferentially as possible going past the midline in the anterior aspect. The incised annulus fibrosis and nucleus pulposus are then removed with a Lexel rongeur (Fig. 37-10). The endplate is then separated from the vertebral body using a Cobb elevator
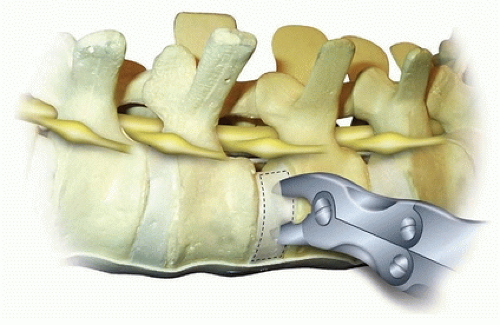
(Fig. 37-11). The elevator is then turned so that it slides down the endplate, as this provides a nice clean plane for complete removal of the endplate in an efficient manner. Each endplate can then be removed with a rongeur after it is completely freed up with a large Cobb. Additional endplate and annulus can then be removed using Zielke curettes, regular curettes, pituitary rongeurs, and Kerrison rongeurs. In severe deformities, the posterior annulus is completely removed, allowing for access to the PLL. The PLL can be removed by initially penetrating the ligament with an angled nerve hook. A Kerrison rongeur is then used to remove the PLL. The author reserves the removal of the PLL for large (greater than 60 degrees) stiff (less than 50% flexibility index) curves since it is usually not necessary in the more typical smaller, flexible curves to achieve fusion and carries some increased neurologic risk. Many surgeons prefer to leave the posterior annulus intact to guard against injury to the nerve roots and intracanal bleeding.
(Fig. 37-11). The elevator is then turned so that it slides down the endplate, as this provides a nice clean plane for complete removal of the endplate in an efficient manner. Each endplate can then be removed with a rongeur after it is completely freed up with a large Cobb. Additional endplate and annulus can then be removed using Zielke curettes, regular curettes, pituitary rongeurs, and Kerrison rongeurs. In severe deformities, the posterior annulus is completely removed, allowing for access to the PLL. The PLL can be removed by initially penetrating the ligament with an angled nerve hook. A Kerrison rongeur is then used to remove the PLL. The author reserves the removal of the PLL for large (greater than 60 degrees) stiff (less than 50% flexibility index) curves since it is usually not necessary in the more typical smaller, flexible curves to achieve fusion and carries some increased neurologic risk. Many surgeons prefer to leave the posterior annulus intact to guard against injury to the nerve roots and intracanal bleeding.
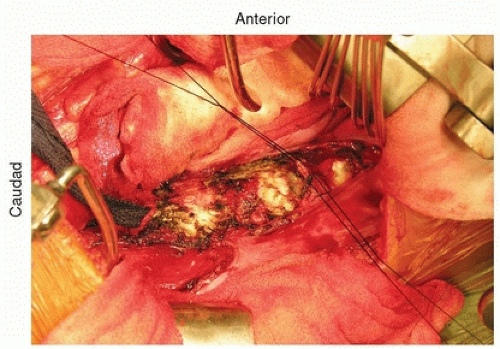 FIGURE 37-7 Ligation of the segmental blood vessels. Two silk sutures have been passed and tied to ligate the vessel. The vessels distally have already been tied off. |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree