Anterior Approach (Traditional Table)
Erik N. Hansen
William J. Hozack
The direct anterior approach (DAA) to total hip arthroplasty (THA) is a well-established surgical technique that has witnessed a recent resurgence in popularity due to a new focus on “minimally or less invasive” surgery. As the anterior approach represents the only intermuscular, internervous approach to the hip, it has the potential to avoid disrupting anatomy and to minimize soft tissue injury compared to other surgical approaches. Moreover, since it is performed in the supine position, it may facilitate patient monitoring during anesthesia, as well as a more accurate assessment of component position and leg length equality. However, for many surgeons who have traditionally been trained using the posterolateral, anterolateral, or direct lateral approach for THA, there are technical aspects of the procedure which present unique challenges and sets of complications. The goal of this chapter is to familiarize the reader with this surgical approach, describe the techniques and technologies available to facilitate the procedure, and to critically evaluate the existing literature of its potential benefits and shortcomings.
This chapter will begin by reviewing the historical background of this surgical approach and the modifications that have been adopted over the years to bring it to its current state. Surgical indications will then be discussed, followed by a review of the relevant anatomy. Then, we will describe in detail the senior author’s preferred surgical technique. We will highlight pearls and pitfalls that have been acquired over 10 years of clinical experience and discuss various techniques and technologies (e.g., fracture table, navigation, fluoroscopy, and offset instruments) that many of the early adopters/proponents developed to facilitate its successful execution. Following that, we will critically review the current literature that compares the DAA to other surgical approaches to the hip in regard to clinical outcomes, soft tissue injury, gait analysis, as well as the unique set of complications encountered during the “learning curve.” Finally, we will assess the indications, limitations, and techniques to perform revision hip arthroplasty utilizing the DAA.
Historical Background
The direct anterior surgical approach to the hip has a long history that dates back over a century. For a more detailed description of this timeline, the authors would refer the reader to a recent article by Rachbauer et al. (1). The original description of this surgical approach has been credited to Carl Hueter, a German surgeon, who published his work Der Grundriss der Chirurgie (The Compendium of Surgery) in 1881, in which he describes the “anterior oblique approach.”
Define the anterior iliac spine and the tip of the greater trochanter. Halve the line between the two points and pierce the tip of the knife in the middle of this line with the blade directed caudally and somewhat inferiorly. The incision is directed parallel to the border of the m. sartorius, but somewhat external. It falls into the muscular interstice between m. sartorius on one side and m. tensor fasciae latae and m. gluteus medius on the other side, and meets the fibers of the m. vastus lateralis, which originate at the anterior face of the trochanter major at the base of the neck. The advantages of the anterior oblique approach are as follows: (1) only one muscle, the m. vastus ext. is injured; for this reason, the leg keeps its tight connections to the pelvis which facilitates rehabilitation; (2) bleeding is so little, that no single ligature has to be done (1,2).
Dr. Marius N. Smith-Petersen (1886–1953), a Norwegian-born American surgeon, popularized this surgical approach in the English-speaking world. He initially described the anterior approach to the hip in 1917 for treatment of congenital hip dislocation. In 1936, he then described an acetabuloplasty operation for multiple hip pathologies including “old slipped upper femoral epiphysis” and “fractures of the neck of the femur with malposition” (3). Simultaneously in other areas of the world, the anterior approach to the hip was being utilized by surgeons for treatment of various other pathologic conditions about the hip, including developmental dysplasia of the hip (DDH) (e.g., Salter, Pemberton), as well as acetabular fractures (e.g., Levine, Judet, and
Letournel). Yet, it was Smith-Petersen’s description of the surgical approach for “mold arthroplasty” in 1949, which is one of the primary reasons his name has become synonymous with the anterior approach to the hip in total joint arthroplasty (4).
Letournel). Yet, it was Smith-Petersen’s description of the surgical approach for “mold arthroplasty” in 1949, which is one of the primary reasons his name has become synonymous with the anterior approach to the hip in total joint arthroplasty (4).
During those and subsequent years, several other surgeons wrote extensively about the anterior approach to the hip for joint arthroplasty. In 1950, the Judet brothers (5) described their early use of this surgical approach for hip hemiarthroplasty using a synthetic plastic femoral head, which they termed a “resection–reconstruction.” In 1978, Wagner (6) utilized the anterior approach for hip resurfacing, as it allowed for “optimum exposure of both acetabulum and femoral head and neck, provides maximum soft tissue release, and allows preservation of the important posterior retinacular vessels.” Yet the modern generation of publications on the anterior approach for hip arthroplasty started in the 1980s, at which time variations on the prior technique were introduced with the aim of facilitating the procedure and improving outcomes. Letournel (7) described a modification to the superficial dissection in 1980 to minimize risk to the lateral femoral cutaneous nerve (LFCN). Several authors began to popularize the idea of “minimally invasive” THA using multiple, smaller incisions rather than a conventional longer, single incision. Berger (8) described the two-incision approach, in which a small anterior approach was used for cup insertion and a second transgluteal incision was used for the insertion of the femoral implant. Light, Kennon, and Keggi published their results using an anterior approach incorporating a small transverse tensor fascia lata (TFL) slit with two or three small skin incisions (9,10). Other authors focused instead their writing on the use of a special orthopedic table to facilitate the execution of the operation. In 1985, the Judet brothers (11) were the first to publish on this topic of adapting a fracture table to total joint arthroplasty. Since then, Siguier et al. (12) and Matta and Ferguson (13) have expanded its popularity. Over the past several years, there has been a resurgence in interest in this approach to THA and an exponential increase in the number of publications on the topic, yet as this prior section has illustrated, the surgical technique is over a century old and there is really little “new” about it.
Indications
Like all other approaches to the hip, the DAA to the hip is a versatile surgical approach that can be used for the majority of reconstructive procedures performed around the hip. The pediatric orthopedic surgeon understands how useful the approach is for the treatment of DDH, including open reduction of a congenitally dislocated hip, various pelvis osteotomies to improve femoral head coverage, and open debridement of a septic hip. The adult reconstructive surgeon knows it is useful for the treatment of femoroacetabular impingement (FAI), symptomatic dysplasia of the hip, as well as advanced degenerative joint disease. With increasing experience with this surgical approach, there are few if any reconstructive procedures that cannot be successfully performed via the DAA.
Femoroacetabular Osteoplasty
Recently, FAI has been recognized as a cause of pain and early arthrosis in the young adult hip. Beck et al. described two distinct subtypes, though it is acknowledged that a mixed pathology may be present in up to 30% to 40% of cases (14). Cam impingement results from a “bump” on the femoral head–neck junction that causes shear damage to the chondrolabral surface with extremes of motion. Pincer-type deformity described overcoverage of the acetabulum due to coxa profunda, protrusion, or acetabular retroversion. Surgical treatment of FAI was classically described employing a surgical dislocation of the hip with trochanteric osteotomy (15). With improvements in technique and instruments, arthroscopic treatment has also gained popularity (16). Yet the morbidity of the surgical dislocation due to potential symptomatic hardware, nonunion of the trochanter, and vascular compromise of the femoral head, as well as the limitations of arthroscopy to adequately visualize the deformity has led others to promote a mini-open femoroacetabular osteoplasty (FAO) (17). The mini-open FAO utilizes the distal portion of the Smith-Petersen approach to the hip to correct the Cam lesion and debride and repair the damaged chondrolabral junction. It also facilitates a dynamic assessment of residual impingement while minimizing surgical morbidity. When the Cam-type deformity extends beyond the posterosuperior retinacular vessels, it is reported to be easier to manage it using the mini-open anterior approach instead of hip arthroscopy, with no risk of femoral head vascular supply (18). Also, direct visualization of the anterolateral acetabular rim allows for an accurate correction of focal overcoverage when it is needed, with no risk of overcorrection and resultant instability as has been reported in hip arthroscopy (19). Despite these promising results, it is recognized that cases of severe retroversion with deficient posterior wall and global overcoverage (i.e., protrusio) are not adequately treated with this approach and represent a contraindication.
Periacetabular Osteotomy
Symptomatic dysplasia of the young adult hip presents a difficult challenge for the adult reconstruction surgeon. The recommended treatment is based largely on the integrity of the articular cartilage. In the absence of advanced degenerative changes, a joint-preserving operation, like the Bernese periacetabular osteotomy, described by Ganz et al. (20) can be utilized with success to achieve a powerful three-dimensional correction of the native acetabulum. The surgical approach was classically described utilizing the Smith-Petersen approach, but recently surgeons have been able to successfully perform the operation with a modified version, utilizing a limited portion of the traditional anterior approach (21). At midterm follow-up, the procedure has been shown to have reasonably good outcomes. Matheney et al. (22) reported a survivorship of 84% at 10-year follow-up, whereas Troelsen et al. (23) reported a hip survival rate of 81.6% at 9.2 years. However, this also indicates that somewhere between 15% and 20% of patients who had a PAO will need a THA at 10 years. The fact that an
anterior approach to the hip was used for the PAO favors the use of the same approach to perform the conversion hip replacement.
anterior approach to the hip was used for the PAO favors the use of the same approach to perform the conversion hip replacement.
Total Hip Arthroplasty
Given adequate training and experience, the DAA is a viable option for all primary and most revision THAs. As with all approaches, the surgeon must go through a learning experience after which a technical expertise naturally develops.
Extended Indications
Total Hip Resurfacing
Total hip resurfacing has been considered a reasonable alternative to THA in the appropriate patient cohort (24). Despite recent concerns regarding adverse reactions to metal-on-metal bearings, some surgeons continue to offer the procedure to younger male patients as a way to maintain bone stock and permit higher levels of activity. Since the surgical exposure is more extensive than that required for THA, approaches that minimize the soft tissue dissection are attractive. Classically, hip resurfacing is performed via an extensile posterior approach or surgical dislocation with a trochanteric osteotomy, which potentially endangers the vascular supply to the femoral head and risks avascular necrosis of the femoral head and early failure of the operation. In response, several authors have advocated using the DAA to perform the procedure (25,26). Kreuzer et al. (25) reported on their early results of the first 51 patients (57 hips) who underwent resurfacing using the DAA and a special table extension. They demonstrated mean HOOSs that were equal to or better than averages reported from a comparative group of patients who underwent THA. However, they documented three atraumatic (5%) and one traumatic (1.8%) femoral neck fracture in their series, leading the authors to conclude that although the DAA may have some clinical benefits, it is technically challenging and surgeons interested in adopting this approach for hip resurfacing should already be very familiar with the approach for THA. Benoit et al. (26) compared the clinical and radiographic outcomes of a consecutive series of 100 resurfacing patients who underwent the procedure using either the DAA with an orthopedic traction table or the surgical dislocation approach (SDA) advocated by Ganz et al. (15). As this represented the first 50 patients undergoing total hip replacement (THR) via the DAA, they also used this series to describe the learning curve of the operation. They found that mean operative time was longer in the SDA group than those in the DAA group (109 vs. 93 minutes, p < 0.01), and that they were able to significantly improve their operative times in the DAA group over the study period (97 minutes for first 25 cases compared to 89 minutes for last 25 patients, p < 0.01). Radiographically, a greater number of cups were placed in the safe zone of 45- to 55-degree abduction in the DAA group (19 vs. 8, p = 0.013), and there was no significant difference in the femoral component position between the two groups. There were no femoral neck fractures in either group, but seven patients in the SDA required further surgery to remove symptomatic hardware. Based on these results, the authors suggest that the DAA is a reasonable alternative to classic surgical dislocation for hip resurfacing if appropriate safeguards are maintained during the learning curve.
Anatomy
The DAA for THR is the only internervous, intermuscular approach to the hip. Superficially, the approach is developed between the sartorius (femoral nerve) and the TFL (superior gluteal nerve) while the deep interval proceeds between the rectus femoris (femoral nerve) and the gluteus medius (superior gluteal nerve). In the absence of complicating factors, no muscles are split or damaged, no tendons are cut and in need of repair. For many reasons, the DAA has the potential to be the ideal surgical approach for THR. However, the approach is not as familiar to most surgeons as the posterolateral, direct lateral, or anterolateral approaches, and therefore a review of the relevant musculotendinous, neurovascular, and bony anatomy is warranted.
Musculotendinous Anatomy
The “Interval”
One of the most common challenges faced by surgeons early in the “learning curve” is correctly identifying the superficial intermuscular interval between the sartorius and TFL. Straying medial risks injury to both the LFCN and potentially the femoral neurovascular bundle depending how far medial one is. Conversely, a superficial dissection which is started too far laterally will necessitate aggressive lateral retraction on the muscle belly of the TFL for the duration of the procedure, which may result in unnecessary, iatrogenic damage to the muscle. Furthermore, approaching the interval too far distally is another common mistake during the “learning curve,” and presents particular challenges during the deep dissection, because of the obscuring bulk of the quadriceps muscle.
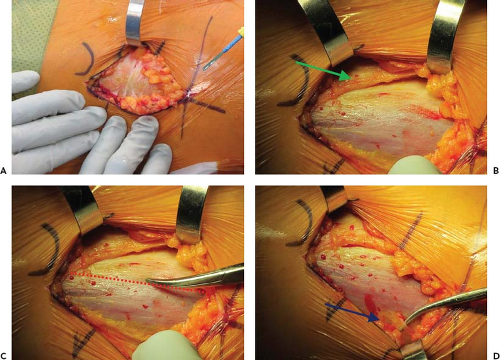
The first structure to identify following the initial skin incision and superficial dissection of the subcutaneous fat is Scarpa’s fascia (Fig. 8.1A). Mistaking this layer for the fascia overlying the TFL is one way to incorrectly identify the interval. However, unlike the fascia overlying the TFL, Scarpa’s fascia is a homogeneous bland color with no lateral perforating vessels. An incision through Scarpa’s fascia should reveal a second and consistent layer of fat just deep to this fascia. Gently sweeping away this layer of fat will allow identification of the true interval (Fig. 8.1B).
Correctly identifying the intermuscular interval of the DAA requires knowing the origins and insertions of the relevant muscles, as well as utilization of other consistent visual anatomic cues. There are three reliable ways to help correctly identify the “interval”:
Identify trajectory of muscle fibers: While both the sartorius and TFL originate from the anterior superior iliac spine (ASIS), the sartorius continues distally toward the pes anserine on the medial proximal tibia, while the TFL continues toward the iliotibial band (ITB) laterally. Therefore, appreciating the differential trajectory of the muscle fibers is one way to help identify the correct location of the “interval.”
White-Red-White: The fasciae overlying the muscles of the interval are of variable thickness and therefore are visually distinct. From lateral to medial, this pattern is reflected in the mnemonic “white-red-white.” The gluteus medius, which is the most lateral muscle, is covered by a dense white fascia (ITB). The fascia overlying the TFL is relatively thin, which allows the red hue of the muscle belly to be easily appreciated, and the fascia transitioning to fat over the sartorius is white in contrast (Fig. 8.1C).
Find the perforating vessels: At the dorsal border of the TFL fascia, there are a consistent set of perforating vessels. Identifying these vessels is another way to confirm that one has correctly identified the TFL and is not too far lateral (Fig. 8.1D).
Separating the sartorius from the TFL is a thin layer of fat, which can be easily entered with blunt dissection using a single finger. Any resistance felt during this maneuver should prompt the surgeon to pause and confirm that the correct interval was entered, since the separation of these muscles should be effortless. As will be described in more detail in the section on the “Author’s Surgical Technique,” rather than entering this fat layer directly, the senior author has been entering the TFL fascia just lateral to the true interval to minimize risk to the LFCN.
Superior/Posterior Capsule and Short External Rotators
Although the exposure of the acetabulum in the DAA is no more difficult than the other approaches, most agree that elevation and preparation of the femur can be the most challenging part of the operation. This is due in large part to a relative lack of experience with this approach in the training programs. The residual superior and posterior capsule as well as the tethering effect of the short external rotators on the proximal femur creates an issue of exposure. Therefore,
a detailed understanding of the location of the short external rotators onto the greater trochanter may assist in understanding the posterior releases necessary for this critical step. At the same time, it may help prevent any unnecessary, iatrogenic injury to these important hip stabilizers. In a cadaveric study comparing the musculotendinous injury resulting from the direct anterior to the posterolateral approach to THR, Meneghini et al. (27) noted that in approximately 50% of the DAAs, a certain portion of the short external rotators were released during the procedure. Although the senior surgeon (WJH) who performed the DAA dissections noted that the cadavers were less supple than expected, this relatively high percentage of releases hints at the ubiquity as well as significance of this step during femoral preparation using this technique.
a detailed understanding of the location of the short external rotators onto the greater trochanter may assist in understanding the posterior releases necessary for this critical step. At the same time, it may help prevent any unnecessary, iatrogenic injury to these important hip stabilizers. In a cadaveric study comparing the musculotendinous injury resulting from the direct anterior to the posterolateral approach to THR, Meneghini et al. (27) noted that in approximately 50% of the DAAs, a certain portion of the short external rotators were released during the procedure. Although the senior surgeon (WJH) who performed the DAA dissections noted that the cadavers were less supple than expected, this relatively high percentage of releases hints at the ubiquity as well as significance of this step during femoral preparation using this technique.
Several cadaveric studies have been performed to better define the anatomy of this area of the proximal femur as well as to describe the relative roles of the various short rotators in preventing femoral elevation. Ito et al. (28) performed an elegant anatomic dissection study to quantitatively define the exact locations of the tendinous attachments of the short external rotators to the medial greater trochanter (Fig. 8.2). Although they reported that there was considerable variation in the attachment of the short external rotators, the attachment site of the piriformis was consistently posterosuperior to that of the conjoined tendon, and the obturator externus attached independently in a fossa located posteroinferior to the other short external rotators. Based on their mapping data, they suggested that preservation of the short external rotators may not always be possible during the capsular release of the DAA (28). In another cadaveric study, Matsuura et al. (29) compared the effect of the differential/sequential releases of the posterior capsule, superior capsule, and obturator internus on the ability to elevate the femur. They demonstrated that the superior capsular release was the most effective of the releases for elevating the proximal femur both in the cadaveric study and a follow-up clinical validation study, and therefore they suggested that additional releases are often unnecessary.
Neurovascular Anatomy
Femoral Neurovascular Bundle
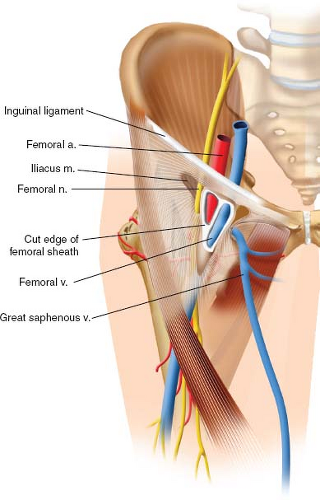
The femoral neurovascular bundle is the single most important anatomic structure at risk in the anterior approach to the hip. It can be easily found traversing the femoral triangle, which is bound superiorly by the inguinal ligament, laterally by the medial border of the sartorius muscle and medially by
the adductor longus (Fig. 8.3). From lateral to medial, the contents of the triangle include the femoral nerve, the femoral branch of the genitofemoral nerve, and the lateral cutaneous nerve of the thigh, as well the three components of the femoral sheath: Femoral artery and its branches, femoral veins, and the femoral canal, which contains lymphatic vessels and lymph nodes. The mnemonic NAVEL can be used to easily remember, from lateral to medial, the configuration of the anatomic contents (Nerve, Artery, Vein, Empty, Lymphatics).
the adductor longus (Fig. 8.3). From lateral to medial, the contents of the triangle include the femoral nerve, the femoral branch of the genitofemoral nerve, and the lateral cutaneous nerve of the thigh, as well the three components of the femoral sheath: Femoral artery and its branches, femoral veins, and the femoral canal, which contains lymphatic vessels and lymph nodes. The mnemonic NAVEL can be used to easily remember, from lateral to medial, the configuration of the anatomic contents (Nerve, Artery, Vein, Empty, Lymphatics).
The femoral neurovascular bundle is at particular risk at two distinct steps during the case: (1) during the superficial dissection, and (2) placing the anterior acetabular retractor. As the DAA begins with superficial dissection on the anterior surface of the TFL, just lateral to the sartorius, it is evident that inadvertently straying medial may put the femoral nerve and vessels at risk. Recognition of the differential direction of the muscle fibers of the sartorius (inserting at the pes anserine—headed toward proximal medial tibia) versus the TFL (inserting in the ITB—headed posterolaterally) may help prevent this mistake. Rather than attempting to dissect directly over the interval, we place our superficial incision a bit lateral to be able to approach the interval through the TFL fascia, as described by Letournel, and stay safely away from both the femoral neurovascular bundle and the LFCN. The second step in the procedure, in which the femoral neurovascular bundle is at risk is when placing the sharp, lighted anterior acetabular retractor over the anterior acetabular wall/column. By keeping the tip of the retractor just over bone, safely under the rectus and iliopsoas, and aiming the tip perpendicular to the ilioinguinal ligament, one can minimize the risk of neurovascular injury (30). Fortunately, reports of major neurovascular injury following direct anterior hip replacement are exceedingly rare.
Lateral Femoral Cutaneous Nerve
In contrast to the rare case of injury to the femoral neurovascular bundle, injury to the LFCN is much more commonly reported. Depending on the degree to which the nerve is damaged (minor stretch vs. transection), the symptoms can range from transient to permanent numbness along the lateral thigh to dysesthesias termed meralgia paresthetica. To minimize the potential of iatrogenic injury during the procedure, one must be familiar with the anatomy and course of the LFCN.
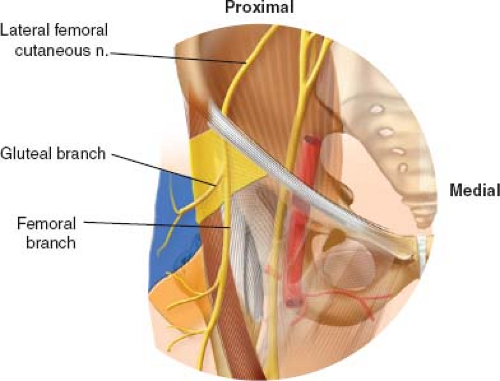
The LFCN is a pure sensory nerve which arises from the dorsal branches of the second and third lumbar ventral rami and emerges from the lateral border of the psoas major. It then courses obliquely, passing medial to the ASIS, under the inguinal ligament and then anterior to the sartorius muscle. The nerve divides into femoral and gluteal branches distal to the inguinal ligament in 62% of cases and proximal to the inguinal ligament in 38% of cases (31). Based on cadaveric work, Ropars et al. (31) reported that in half of all specimens, the femoral branch did not cross the fascia lata muscle, instead descending in the intermuscular plane. Furthermore, they defined a “danger zone” in which the LFCN was potentially “at risk” between 27 and 92 mm below the ASIS (Fig. 8.4). They conclude that there is considerable anatomic variety in the course of the LFCN and recommend positioning the skin incision as lateral and distal to the ASIS as is safely possible to prevent iatrogenic damage to the nerve.
Ascending Branches of Lateral Circumflex Artery
During the deep dissection, the ascending branches of the lateral circumflex artery must be recognized and then ligated or cauterized as they can bleed briskly if inadvertently avulsed. The branches are variable in number and location, but they can routinely be found overlying the fascial layer of the rectus and TFL. After final component insertion, but before closure, it is very important to reinspect these cauterized vessels for any recurrent bleeding.
Bony Anatomy
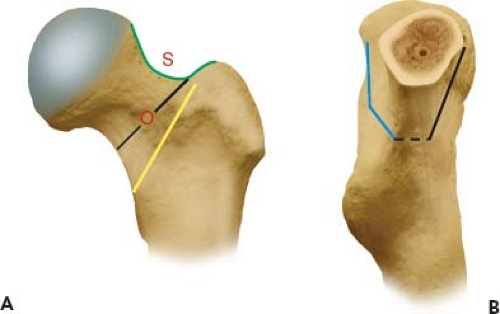
The “Saddle”
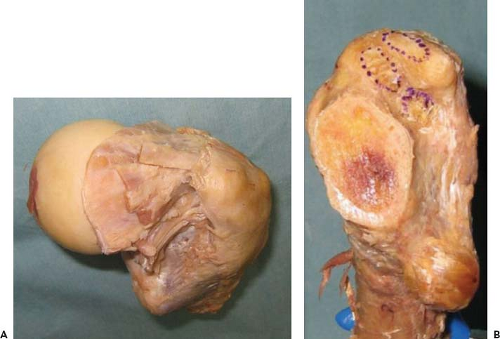
Surgeons who were trained on other approaches to the hip may be unaccustomed to the visualization afforded by the DAA. An important bony landmark of the proximal femur that should be clearly identified on every case is what has been termed the “saddle.” The “saddle” is a concavity which represents the transition from the anterolateral femoral neck to the greater trochanter (Fig. 8.5A). Following capsulectomy, but prior to neck osteotomy, it is critical to clearly define this point as it represents the proximal lateral extent of the neck osteotomy. Depending on the type and design of femoral implant, the distal medial trajectory of the cut may vary from this point. As illustrated in the following figures, the medial exit of the neck osteotomy in the DAA corresponds to the standard cuts made from the posterolateral approach (Fig. 8.5B).
Author’s Surgical Technique
As the DAA to THR has been described extensively in other articles and surgical technique guides, the purpose of this section is to describe the author’s preferred surgical technique refined over the past 10 years, and review various techniques and technologies that have been used to facilitate the operation. Furthermore, as the success of the operation is intimately associated with the perioperative protocols developed at our institution, we will briefly discuss our pre- and postoperative pain and rehabilitation pathways. Finally, we believe the adoption of this technique should be done in a stepwise, responsible manner that includes extensive cadaveric work, observation of established surgeons, and being proctored for the first cases if at all possible. To minimize the potential impact of the learning curve, it is also recommended to start the initial cases on patients who have straightforward anatomy, and only once comfortable, expand one’s indications to patients who may be more technically challenging.
Perioperative Protocol—Pain Management and Rehabilitation
All patients are part of a multimodal pain pathway that starts preoperatively with the administration of a “cocktail” of Tylenol, Lyrica, and Celebrex (32). Unless contraindicated, all patients receive a plain spinal anesthetic of 0.5% ropivacaine. We avoid any long-acting intrathecal narcotics, because the side effects often interfere with early recovery. We do not use Foley catheters, as we believe this delays mobilization and increases the risk of urinary tract infection. All patients receive a second-generation cephalosporin unless they have a documented penicillin allergy.
Postoperatively, patients receive a multimodal pain protocol, which includes use of the following oral medications: Tylenol, Lyrica, and Celebrex. We avoid use of intravenous narcotics, and instead rely on oral narcotics, such as tramadol and oxycodone, if the patient requires that level of pain management. Patients are seen by physical and occupational therapy starting the day of surgery and begin ambulating that afternoon. THA patients will stay one to two nights in the hospital. Because of the anterior approach, patients do not have hip precautions (33). They usually use a walker or crutches for 1 week, followed by a single cane for 2 to 3 weeks, and by 1 month postoperatively walk without an assistive device.
Patient Positioning/Draping
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree