CHAPTER 4
Ancillary Findings Associated With Spasticity
Cindy B. Ivanhoe and Ana V. Durand Sanchez
The ancillary features and findings associated with spasticity and the upper motor neuron syndrome (UMNS) include a spectrum of disabilities. In addition to common problems associated with spasticity, muscle overactivity can potentially affect a wide range of body systems and functions. How these interactions, directly and indirectly, will affect an individual is determined by a range of factors, including diagnosis, duration, age of onset, and chronicity. Inherent in this discussion is how the UMNS affects function across organ systems. For example, skin is affected by positioning and external interventions, such as orthotics and casts. Skin lesions, such as pressure sores, will further impact the presentation of tone because these lesions present a noxious stimulus. Swallowing and breathing are also affected by positioning, weakness, tone, cognition, and coordination. Further, cognition as well as socialization can be affected by postures and dysarthria. Therefore, the integrated effects of the UMNS coalesce to create individual functional issues, and treatment approaches that target one feature of the presentation consequently affect other aspects of overall functioning.
Although the term spasticity is often used to describe a symptom complex that is not limited to spasticity, it is important to untangle the effects of spasticity from other components of the UMNS, including negative features (weakness, decreased dexterity, etc.) and sensory cognitive and behavioral changes. Separating the effects of spasticity without incorporating these other features creates an artificial divide (1). It is also important to consider how spasticity affects the entire body system. For example, does spasticity directly affect the end organ or is the muscle overactivity reducing function in the body system? Both options must be considered. Detrusor-sphincter dyssynergia would be an example of the direct effects of spasticity on an end organ, whereas difficulty with toileting may occur from impaired posture, balance, or motor control. Drooling may result from decreased oromotor control from spasticity, weakness, incoordination, or dystonia (Figure 4.1), and sedation due to medications may increase these baseline impairments even if they reduce tone. Lastly, the combination of spasticity effects on multiple body systems can also impair higher order functional states. Aside from cognitive concerns and psychological issues, sexuality may be affected by restricted muscle movement or contracture, and depression, altered body image, change in role, and self-esteem can significantly affect overall function.
This chapter covers a variety of symptoms, complexes, and body systems that are affected by and are associated with UMNS. Although oral medications are necessary for the management of UMNS, these medications have inherent limitations due to their side-effect profiles, including effects on strength, endurance, and cognition. Maximal functional improvement can often be attained through the combination of botulinum toxin injections, motor point blocks, nerve blocks, and intrathecal baclofen (ITB) therapy coupled with rehabilitation interventions and an understanding of each individual’s goals. Although they may remain undetected by commonly used assessment scales, improvements achieved are frequently noted by patients, caregivers, and clinicians (2).
SENSORY DISTURBANCES
In order to address UMNS, it is necessary to consider the associated sensory issues in that spasticity, a sensorimotor disorder, may be associated with disorders of proprioception, spatial orientation, and other sensory disturbances. Pain is one of the noxious sensory issues associated with spasticity (3,4).
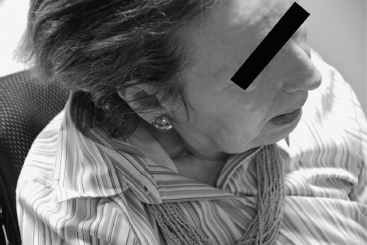
FIGURE 4.1 Cervical dystonia untreated.
Although the mechanisms of pain associated with the UMNS and spasticity are not clearly understood, one hypothesis is that prolonged muscle contraction or activation of the motor pathways stresses the vascular supply or consumes excess oxygen, leading to ischemia. Nociceptor fibers are then activated, thereby contributing to the maintenance of the flexor reflex, and neurotransmitters that contribute to pain are also released. Therefore, pain represents a complication of UMNS that has implications for spasticity, mood, cognition, sexuality, and overall function and quality of life. Pain is a noxious stimulus that can lead to increased spasticity, and the disease itself that underlies UMNS can lead to stresses on the musculoskeletal system (5) and associated weakness, immobility, spasticity, and dystonia. Although interest in assessing and treating pain is becoming increasingly recommended and mandated by regulatory agencies, it is sometimes overlooked by clinicians, especially when the scenario involves someone who is unable to express his or her own needs. Although only limited evidence exists pertaining to the efficacy of baclofen, dantrolene, diazepam, and tizanidine in facilitating functional gains (6), these medication do reduce spasticity; however, often at the cost of alertness, strength, and cognition. Limiting pressure sores in less mobile individuals can also indirectly prevent another potential source of pain as well as another noxious stimulus for spasticity. Oral antispasmodic medications are the most commonly used interventions for spasticity and may have some mild analgesic effects as well (7).
Botulinum toxin injections into spastic, painful muscles have long been noted to decrease pain. They may act to decrease pain in spasticity by decreasing muscle contraction as well as decreasing the release of neurotransmitters associated with pain (3,4). In 2000, the first open-label study was conducted that actually used pain as the primary outcome measure with spasticity considered secondarily (10) and a body of literature currently exists that addresses the effects of botulinum toxins on neurotransmitters that contribute to pain (11).
In multiple sclerosis (MS), no evidence indicates that ITB has a direct impact on pain, although baclofen has been suggested to have some analgesic properties. However, studies show that ITB therapy does improve activity in MS. One study focused on the prevalence and treatment of spasticity in MS found that respondents reported less spasticity and fewer painful spasms when treated with ITB therapy compared with those treated with oral medications alone (8) and satisfaction was greater with ITB therapy when compared to oral medications. The performance scales measured included mobility, hand function, vision, fatigue, cognition, bladder/bowel, sensory, and spasticity (Figure 4.2). ITB can also be used to treat spasticity in cerebral palsy (CP). CP can contribute to deformities in the growing child due to abnormal forces on joints, reduced activity levels, and inefficient biomechanics. These factors are compounded by weakness and balance disturbances, again resulting in pain (9).
Severe spasticity can also lead to complete immobility in these and other pathologies, and result in an overall decline in body systems that can lead to pressure sores, bladder and bowel complications, respiratory compromise, and decreased cognitive interaction.
Pain can primarily be neuropathic and/or musculoskeletal in nature. Complex regional pain syndrome (CRPS), whether type 1 or type 2, can significantly impair function, sleep, and mood. CRPS type 1 involves the sympathetic nervous system, whereas CRPS type 2 involves a specific injury to a nerve. Both syndromes exhibit pain beyond the distribution of the original injury. Therefore, it is essential that clinicians choose medications judiciously in order to avoid side effects that can further negatively impact function. CRPS was formerly known as “reflex sympathetic dystrophy.” It is associated with disabling pain, swelling, vasomotor instability, sudomotor abnormality, and impairment of motor function (12), and the variety of treatments consist of mobilization, antidepressants, steroids, anticonvulsants, and sympathetic blocks (13,14).
Neuroplastic changes have been reported in the sensory and motor pathways in patients with CRPS (15). Central pain syndrome, also known as thalamic pain, can occur after any lesion to the central nervous system (CNS), but this syndrome particularly affects somatosensory pathways, the thalamus, the thalamocortical connections, and the cortex (16). Pain associated with central pain syndrome is typically constant, moderate to severe in intensity, and often made worse by touch, movement, emotions, and temperature changes. Burning pain is the most common presentation, although other descriptions include “pins and needles”; pressing, lacerating, or aching pain; and brief bursts of sharp pain. Individuals may also present with numbness in affected areas. The burning and loss of touch sensations are usually most severe on distant parts of the body, such as the feet or hands. Central pain syndrome often begins shortly after the causative injury or damage but may be delayed by months or even years, especially if it is related to poststroke pain. Diffuse peripheral neuropathies and nerve compressions may arise from trauma and/or repetitive trauma and may need to be distinguished from other pain syndromes. Medication suggestions for these syndromes are relatively interchangeable with variable effects.

FIGURE 4.2 Performance scales (sensory).
Source: Adapted from Ref. (10). Wissel J, Müller J, Dressnandt J, et al. Management of spasticity associated pain with botulinum toxin A. J Pain Symptom Manage. 2000;20(1):44–49.
Early in the rehabilitation process, focus should be placed on interventions that will both limit pain acutely and lead to long-term rehabilitation. For example, prevention of spasms can limit plantar flexion contractures and shearing injury to the skin, and patients displaying extensor tone may benefit from positioning with their legs over a wedge to break up synergy patterns, maintaining their range and limiting their discomfort. Regardless of the nature of the injury, treatments such as standing programs and bed positioning programs serve to decrease hypertonia and the tendency toward more discomfort from potential deformity, spasms, and pressure ulcers among others. This treatment strategy allows for improved stretching, range of motion, pressure relief, breathing, bladder and bowel management, muscle strength, and overall well-being (Figure 4.3).

FIGURE 4.3 (A) Patient 10 years postinjury with recurrent episodes of “asthma.” (B) and (C) After treatment with ITB therapy and a botulinum toxin A every 3 months, patient sees improvement and no longer aspirates. His “asthmatic” episodes have resolved. He begins coughing during eating 2 to 3 weeks before the next set of injections, demonstrating need and efficacy of treatment.
Tone can be affected by positioning and should be a primary assessment task when starting the evaluation of the clinical presentation and treatment plan. Appropriate positioning programs are difficult to maintain in many rehabilitation venues and take the enlistment of the nursing staff (17). Tonic neck and vestibular reflexes modulate tone and can be incorporated into a bed positioning program to correct abnormal, functionally limiting postures. In addition, casting and positioning can diminish tone as can heat or cold, and stretching can diminish tone, albeit temporarily; however, casting a joint to a painful extreme becomes a noxious stimulus of combined physical and emotional pain (Figure 4.4). Therefore, both short-term and longer term benefits must be considered. Nerve root entrapments and direct deafferentation also contribute to pain. In spinal cord injury, incomplete lesions are more likely to be associated with pain. CRPS may be associated with any of the causes of the UMNS, as well as with peripheral injuries. Carpal tunnel syndrome can develop as a result of overuse or of the subsequent deformities that develop from spasticity. Botulinum toxin A has been shown to reduce capsaicin-induced pain and neurogenic vasodilatation without affecting the transmission of thermal pain modalities as measured by the Visual Analog Scale (Table 4.1) (18).
ITB, intrathecal baclofen.
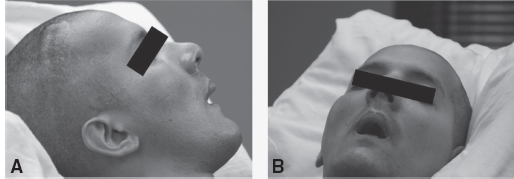
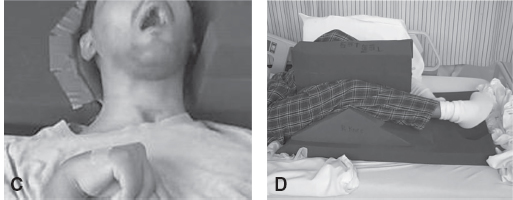
FIGURE 4.4 (A) and (B) Spasticity intervention began 5 years postinjury. The patient’s oropharyngeal swallow was intact. Face remains “stuck” with lips and cheeks in constant contracture. (C) First attempt at LE positioning program. The patient did not tolerate due to restlessness of left LE; he was constantly rubbing against his right LE. Right LE was unable to be flexed. Theraband component was added to the device due to constant adduction over his right LE. The head was unable to be safely positioned to prevent left rotation, as the patient would continually push through anything that was attempted and cry out when it was in place. (D) Second attempt at LE positioning. The patient is tolerating his LE and head position; no restlessness, no crying out, and no resistance to devices. ROM increased to approximately 30° to 40° of right knee flexion.
LE, lower extremity; ROM, range of motion.
Pain in CP is mostly associated with musculoskeletal deformities and conditions related to overuse and arthritis. A combination of fatigue, pain, and weakness develops over time and can be related to deformities, further weakness, and nerve entrapments (19). Positioning programs, energy conservation, analgesics, and therapy modalities of heat and stretching can provide relief (20).
Early intervention with the goal of preventing or decreasing these complications while decreasing tone is preferable. Selective dorsal rhizotomy, ITB therapy, botulinum toxin injections, orthotics, and rehabilitation therapies are all best delivered through a team approach based on the goals of these prescribed interventions.
TABLE 4.1
VISUAL ANALOG SCALE | |
Information point: Visual Analog Scale (VAS) | A VAS is a measurement instrument that tries to measure a characteristic or attitude that is believed to range across a continuum of values and cannot easily be directly measured. For example, the amount of pain that a patient feels ranges across a continuum from none to an extreme amount of pain. From the patient’s perspective of this spectrum appears continuous—the patient’s pain does not take discrete jumps, as a categorization of none, mild, moderate, and severe would suggest. It was to capture this idea of an underlying continuum that the VAS was devised. Operationally, a VAS is usually a horizontal line, 100 mm in length, anchored by word descriptors at each end. The patient marks on the line the point that they feel represents the perception of his or her current state. The VAS score is determined by measuring in millimeters from the left-hand end of the line to the point that the patient marks. |
How severe is your pain today? Place a vertical mark on the following line to indicate how bad you feel your pain is today. | |
No pain l_________________________________l Very severe pain | |
There are many other ways in which VAS has been presented, including vertical lines and lines with extra descriptors. Wewers and Lowe (21) provide an informative discussion of the benefits and shortcomings of different styles of VAS. | |
As such an assessment is clearly highly subjective, these scales are of most value when looking at change within individuals, and are of less value for comparing across a group of individuals at one time point. It could be argued that a VAS is trying to produce interval/ratio data out of subjective values that are at best ordinal. Thus, some caution is required in handling such data. Many researchers prefer to use a method of analysis that is based on the rank ordering of scores rather than their exact values to avoid reading too much into the precise VAS score. | |
Further reading | Ref. (21). Wewers ME, Lowe NK. A critical review of visual analogue scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13:227–236. |
Patient Name: _____________________________________________ Date: _______________
| |
aA 10-cm baseline is recommended for VAS scales. Source: Ref. (22). Acute Pain Management: Operative or Medical Procedures and Trauma. Part 1 AHCPR Publication No. 92–0032; February 1992. Rockville, MD: Agency for Healthcare Research & Quality;116–117. | |
| |
Directions: Ask the patient to indicate on the line where the pain is in relation to the two extremes. Measure from the left hand side to the mark. | |
Source: Ref. (23). Stratton Hill C. Guidelines for Treatment of Cancer Pain: The Revised Pocket Edition of the Final Report of the Texas Cancer Council’s Workgroup on Pain Control in Cancer Patients. 2nd ed.;61–63. Copyright 1997, Texas Cancer Council. Reprinted with permission. www.texascancercouncil.org.
Common causes of pain that are more specific in the acute neurorehabilitation setting include fractures, aggressive therapy interventions, skin breakdown, and urinary tract infections. In one prospective study, 25% of high-risk patients developed adhesive capsulitis. Adhesive capsulitis is associated with impaired consciousness, hemiparesis, duration of postoperative intravenous infusion, age, and depressive personality (24). Botulinum toxin injections appear to decrease the pain of poststroke to a statistically significant degree, but consumption of analgesics does not appear to have a significant effect. In addition, despite injections being limited to the shoulder, a significant decrease in tone can also be detected in the finger flexors (25). This observation is suggestive of the contribution of pain to the presentation of spasticity and/or the decrease in overflow associated with spasticity.
VOICE, SPEECH, AND SWALLOWING
When therapy is considered for a patient with UMNS, it is most commonly physical and occupational; the role of speech and language pathology is often overlooked. However, hypertonicity affects swallowing, cognition, and communication, creating transdisciplinary goals for treatment.
Dysphagia
Dysphagia can present with deficits along different phases of swallowing. If not addressed, complications of aspiration, fevers, and social isolation can become significant. Swallowing assessments can be performed at the bedside or radiographically. Although bedside evaluations do not allow for assessment of the pharyngeal phase of swallowing, these examinations can provide information about oral motor functioning, ability to understand and follow directions, postural concerns, and performance. Specific structures that can be assessed at the bedside include the lips, tongue, palate, larynx, pharynx, and facial musculature. Sensation can also be assessed. Many patients may have difficulty initiating jaw opening to take in bolus or may display a tonic bite reflex or a sensory defensive response. Poor oral hygiene and thrush can further compromise swallowing. A kinematic analysis of swallowing in healthy volunteers showed that the true vocal cords (TVC) respond to bolus consistency in such a way that TVC closure starts earlier and lasts longer with thin liquids as compared to thickened consistencies (26–28).
Fluoroscopic swallowing evaluations or modified barium swallow are commonly performed to allow for a clear definition of the oral and pharyngeal phases of swallowing. The patient is given different consistencies of food, such as thin liquids, pudding, or cookie consistency. Swallowing is monitored for signs of aspiration. Fiberoptic endoscopic evaluation of swallowing (FEES) allows direct visualization of the laryngeal and pharyngeal structures, and can be performed by speech and language pathologists. Once the information is obtained, clinicians can institute appropriate techniques, dietary consistencies, and interventions.
Compensatory strategies for managing dysphagia include postural changes that alter the position of the structures of the oropharynx, thermal or electrical stimulation, textural stimuli, tongue base exercises, different swallowing techniques, and alterations in diet consistency (29).
Oromandibular dystonia presents with involuntary sustained contractions of assorted muscles of the craniopharyngeal area, leading to deviation of the jaw, jaw opening, jaw clenching, as well as abnormal movements and positions of the tongue. These features contribute to difficulties with dysphagia, articulation, pain, and distraction. Similar to the limb muscles, the facial, pharyngeal, and cervical muscles can become shortened, tightened, and painful. Although underestimated, these conditions have been noted in 20% of patients with acute brain injuries (30,31).
Muscles that are closer to the shoulder area but can also affect swallowing include platysma and omohyoid. These should be assessed along with sternocleidomastoid, trapezius, levator scapula and scalenes, which may influence head posture and swallowing function.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree