Parkinson disease is a progressive neurodegenerative disorder characterized by a variety of motor and nonmotor features. This article reviews the problems of postural instability and gait disturbance in persons with Parkinson disease through the discussion of (1) the neuropathology of parkinsonian motor deficits, (2) behavioral manifestations of gait and postural abnormalities observed in persons with Parkinson disease, and (3) pharmacologic, surgical, and physical therapy–based interventions to combat postural instability and gait disturbance. This article advances the treatment of postural instability and gait disturbance by condensing up-to-date knowledge and making it available to clinicians and rehabilitation professionals.
Key points
- •
Parkinson disease (PD) is characterized pathologically by the presence of nigrostriatal dopaminergic cell loss in the basal ganglia, resulting in both motor and nonmotor symptoms. The cardinal features of PD include resting tremor, rigidity, akinesia/bradykinesia, and postural instability and gait disturbance (PIGD).
- •
In addition to basal ganglia disturbance, it has been suggested that activity of the cerebellum and pedunculopontine nucleus is also altered in persons with PD. Given that both brain areas contribute to locomotor control, gait disturbance in persons with PD seems to manifest as a result of abnormal activity within a variety of neural components.
- •
PIGD is a particularly debilitating motor feature of persons with PD, leading to the findings that more than 70% of persons with PD fall during the course of their disease, often resulting in fractures.
- •
Parkinsonian gait is characterized by bradykinesia, stooped posture, high stride-to-stride variability, and, in some persons, freezing episodes. Persons with PD also have decreased stability during both static and dynamic motor tasks.
- •
Although various treatment methods (pharmacologic, surgical, and physical therapy based) have been suggested to alleviate PIGD symptoms with varying degrees of effectiveness, further investigations should pool insights obtained from neurologic, physiologic, and biomechanical perspectives to advance understanding of PIGD in persons with PD and develop effective interventions that specifically address these deficits.
Introduction
Parkinson disease (PD) is a chronic and progressive neurodegenerative disorder that leads to a wide variety of both motor and nonmotor features. PD is characterized pathologically by the presence of nigrostriatal dopaminergic cell loss in the basal ganglia. The cardinal symptoms of PD include resting tremor, rigidity, akinesia/bradykinesia, and postural instability and gait disturbance (PIGD). Of these cardinal motor features, PIGD is one of the most disabling, leading to not only decreased mobility and increased fall frequency but also reduction in quality of life. It is estimated that more than 70% of persons with PD fall during the course of their disease, often resulting in fractures. Physical inactivity as a consequence of frequent falls or fear of falling can also substantially shorten life expectancy for persons with PD. To improve the quality of life for persons with PD, many research studies have evaluated the underlying mechanisms that cause PIGD and have focused on the evaluation of intervention strategies designed to reverse or alleviate PIGD in this population.
This article reviews (1) the neuropathology affecting PIGD in persons with PD, (2) the behavioral manifestation of PIGD, and (3) currently available surgical, pharmacologic, and physical therapy-based interventions to combat PIGD.
Introduction
Parkinson disease (PD) is a chronic and progressive neurodegenerative disorder that leads to a wide variety of both motor and nonmotor features. PD is characterized pathologically by the presence of nigrostriatal dopaminergic cell loss in the basal ganglia. The cardinal symptoms of PD include resting tremor, rigidity, akinesia/bradykinesia, and postural instability and gait disturbance (PIGD). Of these cardinal motor features, PIGD is one of the most disabling, leading to not only decreased mobility and increased fall frequency but also reduction in quality of life. It is estimated that more than 70% of persons with PD fall during the course of their disease, often resulting in fractures. Physical inactivity as a consequence of frequent falls or fear of falling can also substantially shorten life expectancy for persons with PD. To improve the quality of life for persons with PD, many research studies have evaluated the underlying mechanisms that cause PIGD and have focused on the evaluation of intervention strategies designed to reverse or alleviate PIGD in this population.
This article reviews (1) the neuropathology affecting PIGD in persons with PD, (2) the behavioral manifestation of PIGD, and (3) currently available surgical, pharmacologic, and physical therapy-based interventions to combat PIGD.
Neuropathology of PIGD in Parkinson disease
As a result of research spanning several decades, the neuropathology of parkinsonian motor features and their relationships to dysfunction of the basal ganglia and other subcortical structures are becoming increasingly well understood. The basal ganglia are a multifunctional group of subcortical nuclei (the striatum, pallidum, subthalamic nucleus [STN], and substantia nigra) that maintain a vast series of inputs and outputs to transmit signals throughout the brain ( Fig. 1 ). The basal ganglia interact largely with other cerebral motor structures, including the cortex, midbrain, thalamus, and cerebellum, to execute and coordinate voluntary movements such as gait. Pivotal research in the 1980s significantly advanced the understanding of basal ganglia circuitry, outlining not only connections within subcortical structures but also interactions between the basal ganglia and multiple areas of the cortex. Alexander and colleagues expanded on traditional views that proposed that basal ganglia output primarily consisted of connections to the primary motor cortex, and they were among the first to suggest the organization of functionally distinct basal ganglia circuitry, postulating connections between the basal ganglia and multiple cortical areas controlling various functions. For instance, basal ganglia connections with the premotor areas and primary motor and somatosensory cortices are influential on motor function, whereas interactions with structures within the prefrontal cortex and the anterior cingulate are important for cognition.
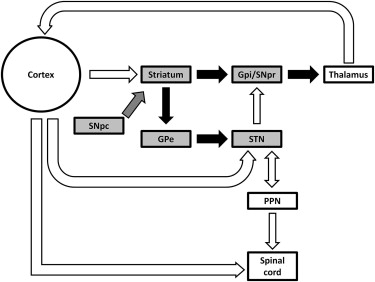
The ideology of functional segregation of interactions between the basal ganglia and cortical structures eventually led to the identification of 3 primary pathways through which the basal ganglia, thalamus, and cortex interact: the hyperdirect, direct, and indirect pathways. When voluntarily initiating movement, these 3 pathways are essential in coordinating the movement through a highly specific selection of available motor programs. First, the hyperdirect pathway is thought to reset the system by suppressing both intended and competing actions by inhibiting activity of the motor cortex. The direct pathway subsequently facilitates the desired motor program for execution of the selected action by exciting the cortex. The indirect pathway then inhibits competing motor programs to efficiently execute the desired movement.
In persons with PD, degeneration of dopaminergic neurons within the pars compacta of the substantia nigra disrupts the normal function of motor program selection by the basal ganglia circuitry, resulting in improper enhancement of desired motor programs and faulty inhibition of competing programs. These irregularities in PD motor control manifest in gait patterns that are characterized by rigidity, bradykinesia, and postural instability (discussed later). Dopaminergic dysfunction often has devastating consequences on gait, because locomotion is influenced not only directly by the aforementioned basal ganglia interactions with the thalamus and cortex but also, perhaps predominantly, through reciprocal circuitry connecting the basal ganglia and the midbrain locomotor region (MLR). In addition to basal ganglia structures, the pedunculopontine nucleus (PPN) of the MLR has recently become a primary target for treatments designed to achieve locomotor improvement.
The PPN is composed primarily of cholinergic neurons, which have been shown to be reduced in some persons with PD, particularly those who experience frequent falling and balance deficits. Like the basal ganglia, the PPN seems to have connections to various motor regions throughout the central nervous system. In addition to reciprocal connections with the basal ganglia, the PPN also interacts with important locomotor control regions in the cerebellum and the spinal cord to influence the initiation, speed manipulation, and termination of gait. Further, recent research on these interactions has produced intriguing results that suggest that dysfunction of the PPN may be a major contributor to the freezing of gait (FOG) phenomenon frequently observed in persons with PD. The effects of PPN stimulation on FOG have become a highly emphasized area of research in the treatment of parkinsonian locomotor deficits.
Because the cerebellum is theorized to share connections with both the thalamus and the PPN, abnormal cerebellar function has been suggested to also contribute to some of the locomotor changes that occur in persons with PD. Hyperactivity of the cerebellum has been observed in persons with PD when performing upper extremity movements and walking. This cerebellar hyperactivity may act to influence cortical mechanisms as necessary compensation for motor control dysfunction of the basal ganglia. However, this abnormal cerebellar function may limit adaptability of gait in PD, because Jayaram and colleagues recently observed that cerebellar depression is proportional to the ability to learn and store different gait patterns. Thus, altered cerebellar function in PD may have important effects on transitional periods during gait in which the rhythmicity and timing must be manipulated to safely and efficiently adapt locomotor patterns. In this way, PIGD in persons with PD seems to be manifested as a multifactorial motor complication involving cortical, subcortical, and cerebellar structures of the brain.
PIGD in Parkinson disease
PIGD is one of the most debilitating symptoms of PD, characterized by spatial and temporal disturbances compared with neurologically healthy adults. Gait disturbances in PD are divided into 2 types: episodic and continuous ( Table 1 ). Episodic gait disturbances (see Table 1 ) occur infrequently and abruptly, in an apparently random manner. These include festination, hesitation of gait, and FOG. In contrast, continuous gait disturbances (see Table 1 ) are persistent and apparent throughout the waking day. For instance, parkinsonian gait is hypokinetic; characterized by decreased gait velocity with short, slow steps ; slowed and decreased arm swing ; increased double-limb support ; and decreased dynamic postural stability. In persons with PD, there is a decreased ability to internally control the center of mass (COM) during self-directed activities such as initiating gait, turning, and stopping, and this inability is commonly associated with falls. Each task involves control of the COM whether in response to unexpected perturbations or to planned transitions between 2 stable postures. This inability to self-regulate postural changes manifests in deficits observed in gait initiation (GI) and gait termination.
| Gait Disturbances | Manifestation of PIGD |
|---|---|
| Episodic | |
| (1) Gait initiation | Decreased COM-COP displacement Increased variability in initial step length Decreased dynamic postural support Decreased mobility Reduced BOS Increased risk of falling |
| (2) Festination | Reduced stride length Increased cadence Decreased gait velocity Increased risk of falling Decreased COG-BOS displacement |
| (3) Gait termination | Decreased gait velocity before termination Increased cadence before termination Abnormal muscle activation Decreased dynamic postural support Increased risk of falling |
| (4) Freezing of gait | Commonly occurring in advanced stage Decreased mobility Decreased dynamic postural support Increased risk of falling |
| Continuous | |
| (1) Gait | Decreased gait velocity, stride length, and time without change in cadence Inability to modulate stride length Decreased arm swing Increased double support time Decreased dynamic postural support Decreased bilateral coordinated gait patterns Increased asymmetry (early stages) Increased gait variability Increased risk of falling |
GI
GI is a functional task that requires a voluntary shift in the center of pressure (COP) from a 2-leg stance into an alternating single-leg stance, thus temporarily reducing the person’s base of support (BOS) (see Table 1 ). These voluntary postural shifts, known as anticipatory postural adjustments (APAs), help generate the momentum needed to initiate forward motion while maintaining balance. Persons with PD have a deficit in maintaining postural stability during the transitional stages between states of static and dynamic equilibrium, such as during GI, gait termination, or turning. The increased difficulty in initiating gait may stem from episodes of start hesitation, which are frequently observed in persons with PD and can occur while on and off dopaminergic medication. GI requires a controlled muscular effort to decouple the body’s COM from the COP to generate forward motion. Patterns of abnormal electromyographic (EMG) activity in the lower extremities has been observed in persons with PD, including cocontraction of agonist and antagonist muscles, increased variability in motor unit recruitment, and decreased frequency and magnitude in first agonist burst. These EMG abnormalities likely stem from deficits in the corticospinal activation of the muscle. Control of the COM and COP during GI has major implications for momentum generation and balance control. The increased displacement of the COM from the COP generates a larger moment arm for the ground reaction forces to drive momentum generation. The transition from double-limb support to an unstable single-limb support decreases dynamic stability by reducing the BOS. During GI, limiting the COM-COP displacement observed in persons with PD seems to compensate for dynamic postural control. Our previous study found that, when alternating foot position from the normal double-limb support stance during GI, persons with PD were able to initiate gait in a similar manner as their neurologically healthy peers. These findings should be expanded to design effective intervention programs that address issues with postural stability during transitions from static and dynamic positions to minimize the risk of falling.
Gait
As mentioned earlier, persons with PD often walk with reduced gait speed, shorter stride length, reduced arm swing, and decreased postural stability (see Table 1 ). Reductions in gait velocity stem from diminished stride length in persons with PD, whereas cadence is typically unaffected. Decreased stride length has been suggested as a compensatory strategy to maintain stability by limiting the displacement of the COM relative to the BOS. Another strategy often implemented by persons with PD to increase dynamic stability is the unique parkinsonian disturbance known as festinating gait, which is characterized by rapid, hypometric steps that minimize displacement of center of gravity relative to the BOS and increase double-limb support time (see Table 1 ). This compensatory gait mechanism is likely generated in response to the inability to control motor processes that simultaneously require regularity, rhythmicity, and symmetry in movements between limbs to create coordinated gait patterns. Often, the ability to synchronize these processes bilaterally are disrupted in PD, resulting in increased gait asymmetry, diminished bilateral coordination, and high stride-to-stride variability. Abnormal gait variability has been shown in all stages of the disease and seems to increase with disease severity. Increased variability in PD locomotion is not limited to steady-state gait; data from our laboratory has shown that persons with PD also show increased variability in stepping during GI compared with their neurologically healthy peers. Previous research has shown a robust relationship between increased variability in both gait and GI and falling in a multitude of healthy and pathologic populations, including PD. Thus, abnormally high gait variability may also be a target for interventions to reduce falling in persons with PD.
Gait Termination
Gait termination is the transition from steady-state walking to a static stance and is a common motor task performed throughout the course of the waking day. Similar to GI, gait termination is achieved through a series of APAs that control the forward progression of the body and dissipate the kinetic energy generated by the forward momentum of walking to achieve a stable static position. In PD, there is a decreased ability to modulate lower extremity muscular activity and ground reaction forces during gait termination (see Table 1 ). Persons with PD have difficulty modulating the rate of force generation because of impaired muscle activation indicated by abnormal EMG patterns. This anomaly is also experienced during periods in which APAs are required to shift the COM in preparation for gait termination. Gait termination involves interlimb coordination that results in an increase in deceleration forces in the leading limb and a consequential decrease in acceleration in the trailing limb. When healthy adults rapidly terminate gait, a set of motor commands are sent to the muscle of the lead and trail limbs. Persons with PD have difficulty switching from one set of motor commands to another. Compared with neurologically healthy age-matched peers, persons with PD are less able to modulate these forces and associated impulses when given an unexpected signal to stop. Given the potential risk of falls when terminating gait, it is imperative that intervention strategies be implemented using gait termination tasks to improve unplanned stopping and decrease the risk of falls.
Falling
Persons with PD have an increased risk of falling compared with their neurologically healthy age-matched peers. Although approximately 30% of older adults fall at least once each year, it has been suggested that this number rises to 70% in persons with PD. Several prospective studies have shown that fall rates in persons with PD exceed those of healthy older adults, and persons with PD are also at a significantly increased risk of suffering recurrent falls. In response to an obvious need to address the mechanisms underlying falling in PD, multiple studies have established several predictors of future falls in persons with PD. Previous falls and disease severity have been shown to be among the most reliable of these predictors of future falling. Generic fall-risk tests have been developed for the general elderly population, although it is uncertain whether these measures are equally sensitive for people with PD. Therefore, there remains a need for research to identify predictors of first falls such that future research may focus on preventing falling before it starts in persons with PD.
FOG
One debilitating episodic phenomenon that occurs in some persons with PD is FOG, the involuntary and sudden cessation of gait that can occur during both the medically On and Off states (see Table 1 ). Bilateral manifestation of FOG is common, but unilateral FOG may also occur, particularly in persons with asymmetrical parkinsonism or in the early stages of disease progression. Duration of FOG episodes varies; some may last only a few seconds, whereas others may continue for 30 seconds or more. FOG is often regarded as a feature of advanced PD and is commonly experienced during step initiation, transitioning through doorways, and turning. FOG episodes have been strongly associated with decreased mobility and a greater loss of independence. Gray and Hildebrand reported that fall risk was increased among persons with FOG, and a strong correlation between falls and FOG has been observed in persons with PD. Although the clinical significance of the relationship between falls and FOG in persons with PD has been well documented, the mechanisms underlying the FOG phenomenon remain an enigma. FOG episodes do not correlate with the cardinal features of parkinsonism such as tremor, bradykinesia, or rigidity, and the pathophysiology leading to FOG in PD is a complex paradigm that is yet to be fully understood. Thus, it is crucial to implement appropriate intervention strategies to reduce FOG in persons with PD, because these interventions would likely minimize falling and improve quality of life.
PIGD deficits cumulatively contribute significantly to decreased mobility, loss of independence, and generally unstable movement patterns in persons with PD. Although PIGD deficits vary among individuals and are often highly task dependent (eg, gait, GI, gait termination, turning), most persons with PD are at least mildly affected by these debilitating motor symptoms. Because a wide variety of deficits regarding movement control and performance during these routine activities of daily living are frequently observed in PD, it is imperative to consider appropriate intervention strategies on a patient-specific basis.
Intervention strategies on PIGD for persons with Parkinson disease
Because there is currently no cure for PD, treatment methods focus on minimizing, delaying, and alleviating motor deficits associated with PD. PIGD symptoms have become a primary target for various therapeutic techniques, because motor impairments have been strongly associated with declining quality of life in persons with PD. A vast body of research into pharmacologic, surgical, and physical therapy–based interventions for PIGD in PD has accumulated over time, identifying treatment methods that target multiple motor deficits with varying degrees of effectiveness ( Table 2 ).
| Intervention Type | Study (Investigators, Year) | N | Participants’ Characteristics | Assessments | Significant PIGD Changes |
|---|---|---|---|---|---|
| Pharmacologic | |||||
| Levodopa | Blin et al, 1991 | 20 | Early to advanced stage (1–17 y after onset), H&Y scale: 1–4 | Spatiotemporal and kinetic gait parameters:
| Gait velocity and stride length were increased after l -dopa intake ( P <.05). Spatial and kinematic parameters (ie, stance duration, swing velocity, double support duration) also improved ( P <.05). In contrast, temporal parameters (stride and swing duration, stride duration variability) were not improved |
| Espay et al, 2012 | 4 | Moderate to advanced stage (6–20 y after onset), with a history of FOG in medicated state | Observation of FOG:
| Medically On state FOG, which worsened in a dose-dependent fashion from the On to the Supra-on state, was observed Two patients also showed FOG during the Off state | |
| Surgical | |||||
| (1) Traditional DBS (STN/GPi) | Rodriguez-Oroz et al, 2005 | 69 | Moderate to advanced stage (6–32 y after onset), H&Y scale: 2–5 | UPDRS-III (assessed both On and Off medicated state):
| Under medically Off state, total motor, gait, and postural stability scores in UPDRS were improved after 3–4 y after surgery ( P <.0001), but these scores significantly worsened from 1 y after surgery ( P <.002) Under medically On state, none of aforementioned scores improved after 3–4 y after surgery. Postural stability score significantly worsened ( P <.0001) |
| (2) PPN-DBS | Stefani et al, 2007 | 6 | Advanced stage based on UPDRS motor score (>70) | UPDRS-III and UPDRS-III subscore (item 27–30) less than:
| Under medically Off condition, the UPDRS-III subscore (items 27–30) was significantly reduced by all 3 types of DBS (all P <.01), but PPN seemed more effective than STN alone. Under medically On condition, PPN-DBS ameliorated global UPDRS-III by 44% and STN-DBS and PPN-DBS improved by 66.4%. More importantly, UPDRS-III subscores (item 27–30) showed the benefit of STN-DBS and PPN-DBS ( P = .05) and PPN-DBS (although n.s.) compared with STN-DBS |
| Physical Therapy | |||||
| (1) RT | Hass et al, 2012 | 18 | Mild to moderate stage (H&Y: 1–3) | GI testing:
| The PRT group showed improvements in the posterior displacement of the COP and the initial stride length and velocity during GI testing (all P <.05) |
| (2) Tai chi | Hackney and Earhart, 2008 | 33 | Mild to moderate stage (H&Y:1.5–3) | UPDRS-III, BBS, TS, OLS, TUG, 6MW and forward/backward gait test:
| TC significantly Improved the BBS when compared with the Control ( P <.05). However, forward walking and OLS did not improve in either group. |
| Li et al, 2012 | 195 | Mild to severe stage (H&Y: 1–4) | LOS test, gait and strength test, functional-reach test, TUG, UPDRS-III, and number of falls:
| Overall, TC participants performed significantly better in LOS test, functional-reach test, and stride length in gait testing than those in the other 2 groups. Gait velocity, muscle strength, TUG, and UPDRS-III were significantly improved in both TC and RT groups compared with the stretching group. However, no significant difference was observed between TC and RT groups in these outcome measures | |
| (3) TT | Herman et al, 2007 | 9 | Mild to moderate stage (H&Y: 1.5–3) | UPDRS-III, ABC, and spatiotemporal parameter during gait testing:
| UPDRS-III, gait velocity, and stride length were improved after 2–3 d after TT (all P <.05), and these benefits were maintained 4 wk after TT (all P <.05). |
Pharmacologic Intervention
The most common pharmacologic treatment used in PD is levodopa (see Table 2 ). Although levodopa has long been considered as the most effective medication to improve some motor features of PD, its effect on PIGD is controversial. Previous studies investigating the effectiveness of levodopa on PIGD in PD have shown conflicting results. For instance, some studies have reported that gait velocity and stride length could be improved by levodopa; however, temporal gait parameters related to rhythm, such as stride duration and its variability, are typically not responsive to levodopa therapy. Blin and colleagues suggested that levodopa may be effective in enhancing activation of muscle groups responsible for gait, which may explain increased stride length and velocity. In contrast, ineffectiveness of levodopa therapy in improving gait variability may be caused by lack of force control during gait, which might indicate that PIGD also results from neurodegeneration within nondopaminergic pathways, possibly cholinergic pathways.
The effects of dopaminergic therapy on FOG in PD are also controversial. Fahn and the Parkinson Study Group reported that high doses of levodopa could delay or prevent the development of FOG in patients with early PD. In contrast, Espay and colleagues reported medically On state FOG in patients with PD taking high doses. Thus, the results are conflicting and suggest that levodopa may successfully reduce FOG episodes in some persons with PD but also induce FOG in others if taken at higher doses. Overall, levodopa does not seem to be an effective option to ameliorate PIGD in patients with PD.
Surgical Interventions
Deep brain stimulation (DBS) is an alternative approach to alleviating a wide variety of parkinsonian motor features, including PIGD, tremor, rigidity, and hypokinesia. DBS is an invasive surgical procedure during which a neurosurgeon implants an electrical lead directly into a target structure within the brain. The lead is connected through an extension wire to a battery-powered neurostimulator that is surgically implanted deep to the clavicle. Once the components are properly placed, the neurostimulator is activated to send electrical pulses to the lead(s) to alter the neuronal activity of the target brain structure and perhaps its downstream circuitry.
The most common locations of DBS implantation in persons with PD are the internal globus pallidus (GPi) and STN, because excessive neuronal activity in these areas has been suggested to cause cardinal motor symptoms of PD, such as tremor, bradykinesia, and rigidity (see Table 2 ). This proposed rationale regarding the stimulation of GPi and STN was supported by nonhuman primate studies reporting improvement in motor function after lesion of these structures in parkinsonian animals and pallidotomy in persons with PD. A previous study reported that STN and GPi stimulation significantly improved Unified Parkinson Disease Rating Scale (UPDRS) motor score, even after 2 years of treatment. Regarding nonmotor PD symptoms, GPi stimulation seems to be effective in alleviating depression, whereas STN stimulation worsened the symptom. In contrast, STN-DBS could reduce the amount of medication usage compared with GPi-DBS. Because of the reported significant medication reduction in human cases and effectiveness of STN lesion for the parkinsonian primate, STN has been used more commonly in many clinical sites. However, recent studies showed that the stimulation of GPi is equally effective as that of STN. More details regarding the effect of STN-DBS and GPi-DBS on parkinsonian symptoms are provided in several review articles of DBS.
A particular concern regarding STN-DBS and GPi-DBS treatment is the diminishing therapeutic effect on PD motor deficits over time. Although it has been suggested that PIGD is improved by both STN-DBS and GPi-DBS within a year after surgery, recent research has indicated that the effects of basal ganglia stimulation on PIGD may diminish significantly over time. St George and colleagues conducted a metaregression of 12 long-term studies of bilateral STN-DBS and GPi-DBS to investigate the effects of DBS on PIGD and other PD cardinal features (ie, tremor, bradykinesia, and rigidity) based on adjusted UPDRS scores. The data revealed that both STN-DBS and GPi-DBS initially improved PIGD, but these symptoms worsened significantly as time progressed. Similar diminishment of DBS effects was not observed on other cardinal PD features, but immediate postsurgery improvements were maintained. These trends are consistent with the previous studies investigating the long-term effects of DBS on PD motor features. These results might indicate that GPi-DBS and STN-DBS indirectly improve gait initially by directly improving rigidity and bradykinesia. However, over time, these stimulation effects become less prominent as the sensorimotor control underlying postural control and gait worsens with progression of PD.
As levodopa treatment and traditional DBS targeting have been shown to minimally ameliorate PIGD symptoms in persons with PD, recent research on stimulation of the PPN has been introduced with some promising results. Improvements in PIGD, reduction of FOG, and reduced risk of falling have been observed as a result of PPN-DBS (see Table 2 ). Plaha and Gill first reported the pronounced effect of PPN-DBS on PIGD in both On and Off medication states in 2 persons with PD. This benefit of PPN-DBS in PIGD seemed to be more pronounced than STN-DBS, as shown by the larger clinical change in UPDRS axial subscore (item 27–30) promoted by PPN-DBS. Moreover, Strafella and colleagues recently reported that PPN-DBS increased regional cerebral blood flow in different subcortical structures, particularly the thalamus, and improved the UPDRS motor score in 1 person with PD. Although further studies with larger sample size, involving carefully selected drug-resistant population and inclusion of persons with and without STN or GPi-DBS, are necessary to better understand the benefits of PPN-DBS on PIGD compared with other DBS targets, it seems that PPN-DBS is more effective in improving PIGD in persons with PD than stimulation of the STN and GPi.
Physical Therapy Interventions
In addition to pharmacologic and surgical treatment, short-term therapy-based and exercise interventions may also be effective and feasible options to delay or reverse the progression of PIGD in persons with PD.
The use of extrinsic (visual or auditory) sensory cues has been shown to improve gait in persons with PD. Cueing is defined as the application of visual or auditory external stimuli associated with the initiation and ongoing facilitation of gait. When an external cue is given, the gait parameters change constantly to adapt to the changing conditions of the environment. McIntosh and colleagues showed that rhythmical auditory stimulation could positively increase the temporal parameters of gait in persons with PD. Cueing techniques such as musical beats, metronomes, or rhythmical clapping have been implemented as strategies for improving gait for persons with PD. Cadence and amplitude are altered when an external auditory cue is present, which suggests that repetitive auditory inputs may simply provide a nonspecific arousal stimulus that improves gait function. Visual cues have shown similar effects on the gait patterns of persons with PD.
Exercise has also been recommended for persons with PD because of the established relationship between exercise and improved cardiovascular and physical function as well as overall health and well-being. There is a large body of empirical evidence that describes the benefits of various exercise interventions (including, but not limited to, progressive resistance training, tai chi [TC] exercise, and aerobic treadmill training) on motor function in persons with PD.
Resistance training in persons with PD has shown robust improvements in physical function and significant lessening of PD motor symptoms (see Table 2 ). Dibble and colleagues reported that a 12-week high-force eccentric resistance training program significantly improved muscle size and force production of the quadriceps femoris muscle, which was associated with increased scores in strength-related gait tasks (6-minute walk and stair ascend/descend tests) in 10 persons with PD. To further understand how the improvement resulting from resistance training positively affects PIGD in persons with PD, Hass and colleagues recently investigated the effect of 10 weeks of progressive resistance training on GI in persons with PD. They observed more efficient APAs and increases in initial step length and velocity in persons with PD after resistance training. These results were coupled with significant improvements in lower extremity muscle strength. Thus, these findings might suggest the potential of progressive resistance training to reduce PIGD by improving muscle strength in persons with PD, although further investigations with larger sample sizes are needed.
Tai chi (TC) has been also shown to be effective in improving balance and gait in healthy older adults. Because of the safe and simple nature of TC training, TC has gained attention as a possible intervention to improve PIGD in persons with PD as well (see Table 2 ). However, research on the effects of TC exercise interventions on PIGD in persons with PD has shown conflicting results. Li and colleagues recently reported that gait velocity significantly improved after 24 weeks of TC training. Kim and colleagues also reported a significant improvement in GI performance in persons with PD after participation in a 12-week TC intervention. In contrast, multiple studies have failed to observe any improvements in gait after long-term TC training. Most TC studies in PD populations have evaluated motor function using clinical scales such as the Berg Balance Score and UPDRS. Therefore, further studies should implement more objective biomechanical evaluation of motor performance to further investigate the effectiveness of TC on PIGD in persons with PD. Refinement of TC training regimens also seems to be essential for maximizing improvements in PIGD. Thus, future research should focus not only on whether or not TC improves PIGD in persons with PD but also how TC interventions can be designed so that they are optimally effective for persons at various stages of the disease.
Aerobic exercise, particularly chronic treadmill training (TT), has also been suggested to effectively ameliorate parkinsonian locomotor deficits (see Table 2 ). Overground gait has been shown to improve after TT, as shown by increases in stride length and gait speed and step length as well as decreases in double-limb support time and stride-to-stride gait variability. Some studies have also suggested that fall incidence and fear of falling were reduced after TT. Post-TT improvement of global parkinsonian disability as evaluated by the UPDRS has also been commonly observed. There seems to be a mechanism specific to TT that drives the observed gait improvements, extending beyond the cardiovascular and physiologic benefits of generalized aerobic exercise, because overground gait training has not yielded similarly promising results in this population. It has been hypothesized that persons with PD are able to use the proprioceptive feedback from the continuous movement of the treadmill belts to facilitate alterations and improvements in their gait patterns.
Recent research has begun to explore the effectiveness of other forms of TT on locomotion in persons with PD. Previous studies have suggested that body weight–supported TT may induce similar improvements in gait compared with conventional TT in PD. Moreover, this type of treadmill exercise may be beneficial to persons at more advanced disease stages who may be unable to tolerate the intensity of repetitive conventional treadmill exercise. Split-belt TT also represents a novel approach to gait rehabilitation in that the walker must adapt and learn a new gait pattern while undergoing aerobic exercise. Although research regarding split-belt TT in PD is still in its infancy, we speculate that some of the improvements in gait symmetry and global gait function observed in persons after stroke as a result of these interventions may also substantially benefit persons with PD.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








