Adrenal Cortex
Patricia A. Donohoue
NORMAL ADRENAL DEVELOPMENT AND STEROID HORMONE SYNTHESIS
The fetal adrenal cortex develops from coelomic mesothelium in proximity to the developing bipotential gonads. The cortex then separates from the gonads, which eventually migrate to their adult positions. For this reason, rests of adrenocortical tissue may appear along the paths of migration or near or within the gonads in adults. By the sixth week of gestation, steroid-producing cells appear in the adrenal cortex, and by the tenth week, the fetal zone (comprising 80% of the total volume) and the adult (definitive) zone are producing steroid hormones
under the stimulatory control of adrenocorticotropic hormone (ACTH) stimulation. The fetal zone, whose major products are estrogen and androgen precursors, begins to degenerate by the eighth month of gestation. At that time, the adult zone begins to develop and then differentiate into a mature adult cortex that secretes the three families of steroid hormones: mineralocorticoids, glucocorticoids, and androgens. This differentiation is not completed until the child is approximately 3 years of age.
under the stimulatory control of adrenocorticotropic hormone (ACTH) stimulation. The fetal zone, whose major products are estrogen and androgen precursors, begins to degenerate by the eighth month of gestation. At that time, the adult zone begins to develop and then differentiate into a mature adult cortex that secretes the three families of steroid hormones: mineralocorticoids, glucocorticoids, and androgens. This differentiation is not completed until the child is approximately 3 years of age.
The adult adrenal cortex constitutes approximately 90% of the mature gland and is composed of three zones. The outermost zone, the zona glomerulosa, accounts for 15% of the cortical volume and is the site of mineralocorticoid synthesis. The zona fasciculata constitutes 75% of the cortex, and the reticularis (the innermost zone) constitutes 10%. The zona fasciculata and zona reticularis are one functional unit, involved in glucocorticoid and androgen biosynthesis. The zona reticularis is thought to secrete steroids under basal conditions, and the zona fasciculata stores lipids for stress steroidogenesis.
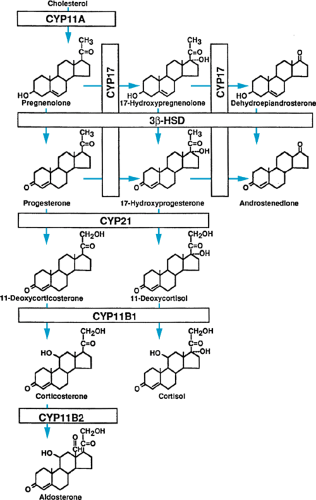
All three groups of steroid hormones are produced from cholesterol, which is supplied by the circulation or produced endogenously. Under the stimulation of the anterior pituitary hormone ACTH, cholesterol is converted to pregnenolone. This conversion is the rate-limiting step in steroid hormone biosynthesis. Pregnenolone serves as the precursor for all three families of adrenal steroid hormones (Fig. 381.1).
Pituitary ACTH release is controlled by the hypothalamic peptide, corticotropin-releasing hormone (CRH). ACTH secretion also may be under the influence of the immune system. Normal ACTH secretion occurs in a diurnal pattern, which results in the normal diurnal fluctuation in serum cortisol levels: highest in the early morning and lowest in the evening. The serum cortisol level completes the feedback loop by stimulating (low cortisol levels) or suppressing (high cortisol levels)
hypothalamic CRH secretion and pituitary ACTH secretion. Serotonin stimulates and norepinephrine inhibits hypothalamic CRH secretion. The daily cortisol secretion rate initially was determined to be 12.1 ± 3 mg/m2 of body surface area (the total daily cortisol production increasing with growth), and this value still is widely accepted. More recent studies, employing different techniques, suggest a lower but more variable secretion rate. Based on the average daily production of cortisol and the potencies of various pharmacologic glucocorticoid preparations, recommendations can be made about the average daily dose of each that would be required for physiologic replacement (Table 381.1). Many preparations also have a mineralocorticoid effect (Table 381.2). In times of physiologic stress (fever or other illnesses), the recommendation is that the oral cortisol replacement doses be increased by threefold.
hypothalamic CRH secretion and pituitary ACTH secretion. Serotonin stimulates and norepinephrine inhibits hypothalamic CRH secretion. The daily cortisol secretion rate initially was determined to be 12.1 ± 3 mg/m2 of body surface area (the total daily cortisol production increasing with growth), and this value still is widely accepted. More recent studies, employing different techniques, suggest a lower but more variable secretion rate. Based on the average daily production of cortisol and the potencies of various pharmacologic glucocorticoid preparations, recommendations can be made about the average daily dose of each that would be required for physiologic replacement (Table 381.1). Many preparations also have a mineralocorticoid effect (Table 381.2). In times of physiologic stress (fever or other illnesses), the recommendation is that the oral cortisol replacement doses be increased by threefold.
TABLE 381.1. GLUCOCORTICOID DOSAGES | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
Most cortisol circulates in the blood bound to cortisol-binding globulin (CBG), also known as transcortin, an alpha-globulin secreted by the liver. The free fraction of cortisol is the biologically active hormone. Estrogens increase levels of CBG, and liver disease and the nephrotic syndrome are associated with decreased levels of CBG. However, levels of free cortisol are unaffected by these conditions.
The major physiologic metabolic effects of cortisol are glycogen synthesis, gluconeogenesis, fat catabolism, and protein catabolism. At high levels, glucocorticoids induce a wide variety of metabolic changes, including immunosuppression, osteoporosis, glucose intolerance, increased gastric acid secretion, and altered central nervous system (CNS) function, resulting in psychiatric symptoms.
Mineralocorticoid (e.g., aldosterone) secretion is controlled mainly by the renin-angiotensin system and also by serum potassium levels. The cells of the zona glomerulosa, which have specific membrane receptors for angiotensin II, secrete aldosterone and its precursors. Stimulation of the adrenal cortex with ACTH produces only a transient increase in levels of aldosterone. The average daily aldosterone secretion rate is not related to body surface area and is similar for infants and adults (approximately 100 μg/day). The major physiologic effect of mineralocorticoids is exerted at the level of the distal convoluted tubule, where they promote retention of sodium and excretion of potassium.
TABLE 381.2. RELATIVE POTENCIES OF CORTICOSTEROIDS | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||
The factors that control secretion of adrenal androgen are not understood as well. In prepubertal children, the production of adrenal androgens is very low. At puberty, their production increases and normal adrenarche occurs. The most potent adrenal androgen is androstenedione, which is converted outside the adrenal gland to the more potent androgen testosterone. At very high levels, dehydroepiandrosterone, a weak androgen, may exert androgenic effects. In pubertal and adult males, the adrenal gland contributes little to the total production of androgen. However, in pubertal and adult females, at least 50% of the circulating testosterone is derived from adrenal androstenedione.
TESTS OF ADRENOCORTICAL FUNCTION
Static Tests
The static tests of adrenocortical function provide important but limited information and often must be accompanied by dynamic testing in the diagnostic evaluation of adrenal disorders. Normal values for some levels of adrenal steroids and responses to dynamic tests are given in Table 381.3.
The level of serum cortisol is measured by immunoassay and is interpretable only if the time of day that the sample was obtained is known. If the cortisol level is subnormal at 8 AM, the time of a normal peak, hypocortisolism is suspected. However, this test does not discriminate among primary adrenal failure, ACTH deficiency, or an enzymatic defect in the biosynthesis of cortisol. A low level of cortisol late in the day is normal and has little value in the assessment of adrenal failure. However, if the level is elevated in a nonstressed patient, it may indicate absence of the normal pattern of diurnal variation often seen in Cushing syndrome. Because the level of serum cortisol rises briskly in response to such stresses as fever, trauma, surgery, fear, or anxiety, single determinations of levels of cortisol cannot be used reliably to diagnose hypercortisolism.
Determination of the level of serum ACTH is useful only if it is accompanied by other tests of adrenal function. The diurnal variation in levels of cortisol is preceded by similar fluctuations in levels of ACTH. An extremely elevated level of ACTH in the setting of subnormal levels of serum cortisol suggests primary adrenal failure. However, because ACTH mediates the rise in serum cortisol in response to stress, its levels vary widely.
Mineralocorticoid status is assessed by measuring concentrations of serum electrolytes and plasma renin activity (PRA), which is elevated in mineralocorticoid deficiency. PRA is a measure of the rate of conversion of angiotensinogen to angiotensin I. Such factors as blood pressure, posture, intake of sodium, and renal function affect PRA and must be considered in the interpretation of the test result. The PRA assay may be useful in monitoring the adequacy of mineralocorticoid replacement therapy. A direct renin assay also is now available. Levels of plasma or urinary excretion of aldosterone and deoxycorticosterone also are useful for assessing secretion of mineralocorticoids.
TABLE 381.3. NORMAL PLASMA AND URINARY STEROID HORMONE LEVELS WITH STATIC AND DYNAMIC TESTS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree