Acute Respiratory Distress Syndrome
M. Michele Mariscalco
Acute lung injury (ALI) is a syndrome of lung inflammation and increased vascular permeability. It is associated with clinical, radiologic, and physiologic abnormalities that cannot be explained by left atrial hypertension. The term acute respiratory distress syndrome (ARDS), formerly known as adult respiratory distress syndrome, is reserved for the most severe end of the spectrum of acute lung injury. It can occur in adults, children, and infants. The syndrome first was described in 1967, by Ashbaugh and Petty, who reported a series of 12 patients (one 11-year-old) suffering from a syndrome similar to hyaline membrane disease in newborns. In 1994, the American-European Consensus Conference (AECC) recommended the following definition for ARDS: (a) acute onset of respiratory distress; (b) hypoxemia (PaO2/FIO2 of 200 mm Hg or less, regardless of the level of positive end-expiratory pressure [PEEP]); (c) detection of bilateral pulmonary infiltrates on the frontal chest radiograph; and (d) no clinical evidence of left atrial hypertension or a pulmonary capillary wedge pressure of no more than 18 mm Hg. Patients who have a PaO2/FIO2 level of 300 mm Hg or less are considered to have ALI.
EPIDEMIOLOGY
A determination of the incidence of ALI, ARDS, and acute respiratory failure (ARF) has been problematic even in adult patients because of the difficulty in defining ALI, ARDS, and ARF in population-based studies. At present, the incidence of ALI is estimated at 22 to 87 cases per 100,000 each year, of which 75% of such cases fit the AECC definition of ARDS. The data are more limited in children and, therefore, even more problematic. In a study using the 1999 hospital discharges from six large states (22% of the U.S. population), the overall average incidence of mechanical ventilation in children (a surrogate marker for ARF) is 47 cases per 100,000 population each year, with 73 cases per 100,000 each year in the 1- to 4-year-old group and 30 cases per 100,000 in the 5- to 9-year-old group. In this study, 64% of these cases were nonsurgical. Note that defining ALI or ARDS in this patient population was not possible. Of the nonsurgical cases, 35% were caused by severe sepsis, 57% by pneumonia, and 10% by asthma. In a 24-month surveillance study conducted in the pediatric intensive care unit (ICU) at the Children’s Hospital of Pennsylvania in the early 1990s, 2.7% of children admitted had ARDS diagnosed during their stay in the ICU. ARDS occurred in approximately 12% of children admitted to the pediatric ICU for sepsis, viral pneumonia, smoke inhalation, or drowning. A low incidence (less than 3%) was observed in children admitted for pulmonary contusion or multiple trauma.
In adult studies, shock from any cause has been associated with ARDS, as have surface burns, smoke inhalation, infectious and aspiration pneumonia, and disseminated intravascular coagulopathy. Current evidence supports that 115,000 people with sepsis each year will develop ARDS. Sepsis is the primary etiology in adults for ARDS. Other causes include drug overdose, cardiopulmonary bypass, fat embolism, high-altitude exposure, toxic gas inhalation, pancreatitis, and massive transfusion. At present, death rates from ARDS are less than 40%, although one recent study of 17,000 adult ventilated patients demonstrated a 52% mortality rate. Important to highlight is that those with ARDS who die, do so from their underlying illness, sepsis, or multiorgan dysfunction and not from their lung injury. The ARDS mortality rate in adults in the late 1970s was 90%. Death from ALI is calculated to amount to 17,000 to 43,000 deaths per year (deaths from acute myocardial infarction and breast cancer are approximately 200,000 and 40,000, respectively, in comparison). Mortality rates in children who are ventilated have been reported to be between 1.6% and 14%, depending on whether rates were based on patients who were followed prospectively for respiratory failure or were based on epidemiologic data. This rate is quite low compared with 30% in adult patients in a 2002 prospective study. Mortality rates in children with ARDS range from 40% to 75%, although more recent reports suggest that it may be as low as 30%.
PATHOGENESIS
No unifying hypothesis for the pathogenesis of ARDS exists. Experimental data from various studies indicate that the activation of complement causes granulocytes to adhere to and damage pulmonary microvascular endothelial cells via the release of lysozymes and toxic oxygen radicals. Increased permeability of pulmonary capillaries results. Studies of humans with ARDS using bronchoalveolar lavage have demonstrated large concentrations of neutrophils, along with the presence of such inflammatory cytokines as tumor necrosis factor and interleukin-1. Other mediators, such as the metabolites of arachidonic acid, prostaglandins, thromboxanes, and leukotrienes, also have been implicated in the increase in vascular permeability and pulmonary artery changes. Evidence does suggest that pulmonary endothelial injury can occur via neutrophil-independent mechanisms, as is the case in oxygen toxicity or endotoxin-induced ARDS models. Clearly, many complex permutations underlie the phenomenon of ARDS, and therapy must be directed at more than one mechanism if morbidity and mortality are to improve further. Despite a number of clinical trials using “antiinflammatory” therapies, none has proved to ameliorate the course of the disease. Only the use of ventilation strategies has demonstrated improved benefit.
CLINICAL MANIFESTATIONS AND COMPLICATIONS
The clinical course of the patient with ARDS varies, depending on the inciting event. Once direct or indirect injury has
occurred, the affected patient usually experiences a latent period during which the respiratory status appears stable. Tachypnea and tachycardia develop primarily during the first 12 to 24 hours. As the syndrome progresses, the work of breathing increases dramatically, with nasal flaring and intercostal and accessory respiratory muscle activity developing. Lung compliance worsens. Auscultation of the chest reveals either no abnormality or high-pitched end-expiratory crackles throughout the lung fields. In some patients, ARDS resolves completely in the acute phase. In others, it progresses to fibrosing alveolitis with persistent hypoxemia, increased alveolar dead space, and a further decease in lung compliance. Pulmonary hypertension secondary to obliteration of the pulmonary capillary bed may be severe and lead to right ventricular failure. Once ARDS and respiratory failure occur, affected patients require a prolonged course of mechanical ventilation (mean of 8.8 days) and have a prolonged length of stay in the ICU (14.3 days) and in the hospital. (24.5 days). Patients may develop severe fibrotic lung disease requiring prolonged mechanical ventilation.
occurred, the affected patient usually experiences a latent period during which the respiratory status appears stable. Tachypnea and tachycardia develop primarily during the first 12 to 24 hours. As the syndrome progresses, the work of breathing increases dramatically, with nasal flaring and intercostal and accessory respiratory muscle activity developing. Lung compliance worsens. Auscultation of the chest reveals either no abnormality or high-pitched end-expiratory crackles throughout the lung fields. In some patients, ARDS resolves completely in the acute phase. In others, it progresses to fibrosing alveolitis with persistent hypoxemia, increased alveolar dead space, and a further decease in lung compliance. Pulmonary hypertension secondary to obliteration of the pulmonary capillary bed may be severe and lead to right ventricular failure. Once ARDS and respiratory failure occur, affected patients require a prolonged course of mechanical ventilation (mean of 8.8 days) and have a prolonged length of stay in the ICU (14.3 days) and in the hospital. (24.5 days). Patients may develop severe fibrotic lung disease requiring prolonged mechanical ventilation.
DIAGNOSIS
An early study of extracorporeal membrane oxygenation (ECMO) in ARDS, completed in the 1970s, was critical to furthering our understanding of the histopathology of the disease. ARDS often is progressive and is characterized by distinct stages having different histopathologic, clinical, and radiographic findings. In the acute phase, interstitial and then alveolar edema develops within the first 24 to 96 hours after injury. Erythrocytes, neutrophils, and macrophages begin to move into the interstitium and alveolus (Fig. 454.1). The thin, type I alveolar cells that form the normal epithelial lining of the lung are destroyed, leaving denuded basement membranes. The epithelial damage appears to be more severe at this stage than is the damage to the capillary endothelium. Within 72 hours of the injury, type II alveolar cells proliferate dramatically (Fig. 454.2). These cells are thick, enzymatically active, and responsible for the production of surfactant. Hyaline membranes form over the denuded alveolar surface (Fig. 454.3). In the proliferative phase, during the next 3 to 10 days, the alveolar septum becomes markedly thickened and is infiltrated by proliferating fibroblasts, plasma cells, leukocytes, and histiocytes. The first endothelial changes can be seen as irregularities of the luminal surface. However, significant capillary injury can occur with little morphologic change in the endothelial cells. By the end of the first week, fibrotic changes may develop in both the alveolar septa and the hyaline membranes. The alveolar structure no longer is recognizable (Fig. 454.4). This diffuse alveolar damage is the pathologic hallmark of ARDS.
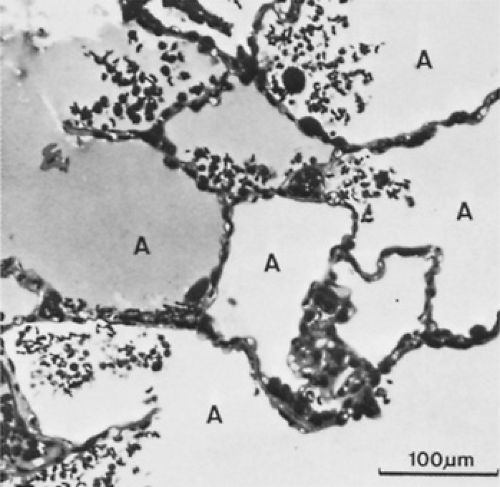 FIGURE 454.1. Light-microscopic view showing pulmonary edema and alveolar spaces. (A) inhomogeneously filled with proteinaceous fluid. The alveolar walls are edematous, and the capillaries are congested. Red blood cells and leukocytes have spilled into the alveolar spaces. (Reproduced with permission from Bachofen M, Weibel ER. Structural alterations of lung parenchyma in the adult respiratory distress syndrome. Clin Chest Med 1982;3:35.)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|




