18
Acute Osteochondral Defects in the Knee
Despite surgical and technologic advancements, the treatment of osteochondral defects continues to challenge orthopaedic surgeons. Articular cartilage lesions are common and have been reported in 63% of over 31,000 arthroscopic procedures in one series.1 Although 20% have been documented to extend to the subchondral bone at the time of surgery, few studies exist delineating the actual incidence of osteochondral lesions of the knee following acute injury. This is complicated by the lack of differentiation in much of the literature between osteochondritis dissecans and other osteochondral lesions or fractures. The injury may go undiagnosed after subtle injury, or if severe enough, may cause significant effusion, hemarthrosis, and dysfunction. Although the natural history of isolated osteochondral defects is unknown, it is hypothesized that untreated lesions may progress and lead to the development of generalized osteoarthritis.2
Osteochondral injury sets off a cascade of events within the joint that extend beyond the immediate zone of injury and stimulate chondrocyte death. Chondrocyte death and presumed progression to degenerative joint disease may occur primarily as a result of mechanical trauma and altered biomechanics, or as a consequence of exposure to known mediators of chondrocyte loss including hemarthrosis, reactive oxygen species, inflammatory cytokines, and matrix degradative enzymes.3–6 Injury due to severe impact or repetitive trauma decreases proteoglycan concentration in the matrix, increases tissue hydration, alters the fibrillar organization of collagen, and alters the ability of chondrocytes to perform synthetic and degradative homeostatic functions. Chondrocyte preservation is paramount in preventing subsequent disability as native hyaline cartilage possesses superior durability and wear characteristics and has a poor capacity for healing.7,8 Recent advances in understanding the complex intracellular biochemistry as well as joint and tissue biomechanics may improve the outcomes of existing treatment modalities that have failed to consistently restore osteochondral defects with native hyaline cartilage and promote “edge-healing.” Current operative treatment includes debridement and stabilization, stimulation of intrinsic repair mechanisms, and repair or transplantation of chondral and osteochondral tissue.
Acute mechanical injury within the knee may produce osteochondral defects by three distinct mechanisms: impaction, shear, and avulsion. Under normal physiologic conditions, articular cartilage is able to accommodate external loading by viscoelastic deformation, which serves to effectively increase joint contact area and decrease contact stresses.9 Loading and deformation generate a combination of tensile, compressive, and shear stresses within the tissue, which, if exceeded, lead to failure. Impact loading has been shown to instigate both acute and progressive damage to articular cartilage of the knee joint in animal models.10–15 Animal studies have also found that repetitive loading of articular cartilage in knees results in subchondral plate fractures and tidemark advancement that is mechanically less compliant.12 The proteoglycan matrix and water content are the primary constituents of articular cartilage responsible for resisting compressive loads. High compression forces manifest clinically as surface cracks in articular cartilage, which can propagate to the tidemark and result in delamination and production of an osteochondral fragment.16,17 Such forces can occur acutely, as when the distal femoral condyles strike a dashboard during a motor vehicle accident, or secondary to repetitive microtrauma and progressive loss of the intrinsic viscoelasticity of hyaline cartilage. Generally speaking, high-energy compressive forces produce greater injury to articular and superficial tangential zone (STZ) chondrocytes, whereas low-energy, low-velocity compressive forces produce selective injury to the deeper structures (e.g., deep zone of cartilage and tidemark).14,16,18
Shear forces can also generate damage to articular cartilage by creating surface irregularities, but place the greatest strain at the interface between calcified cartilage and subchondral bone. Type II collagen, which comprises 90 to 95% of total collagen in articular cartilage, is the primary constituent responsible for resisting shear stress.9,19 Normal shear forces are generated under physiologic conditions with femoral rollback or in athletes involved in pivoting sports. Abnormal shearing can occur in athletes involved in sports that require aggressive pivoting, following patellar dislocation, or in the setting of ligamentous laxity or meniscal pathology. The vertical orientation of collagen fibrils at the tidemark are poorly suited to resist shear stresses and result in progressive chondral delamination and potentially osteochondral fragmentation. Experimental models of osteoarthritis that simulate nonphysiologic shear forces following anterior cruciate ligament (ACL) transection or meniscectomy show evidence of acute and chronic forms of cartilage degeneration.5,20–26
When cartilage is loaded in tension, collagen and proteoglycan molecules align and stretch along the axis of loading. Surface zone articular cartilage is much stiffer than middle and deep zone cartilage, and this is attributed to the parallel orientation of collagen fibers at the articular surface. Collagen cross-linking is the primary determinant of the tensile properties of cartilage, and any agents such as proteolytic enzymes that disrupt this architecture dramatically reduce the tensile strength of articular cartilage.27 Avulsion injuries to articular cartilage, such as in the setting of tibial eminence fractures, occur when tensile forces exceed the tensile modulus.
The amount of bone that is part of an acute osteochondral fragment is dependent on the mechanism of injury, with shearing types of forces generally producing a smaller percentage of attached subchondral bone and avulsion types of forces producing a greater percentage of subchondral bone. In addition, the amount of bone that is attached to the cartilage fragment is dependent on the age of the patient. In the immature skeleton, where there is no calcified zone, shearing forces are transmitted directly to subchondral bone producing a higher percentage of bone within the fragment. The quantity of bone in the osteochondral fragment is also dependent on bone density and the quality of overlying hyaline cartilage. In summary, mechanical trauma can manifest as microdamage to otherwise intact cartilage, damage to cartilage with intact subchondral architecture, or fracture of the cartilage and underlying subchondral bone.
 Classification
Classification
Osteochondral fragments have been well recognized and described for over 250 years, whereas isolated chondral fragment have been reported only in the past 20 years.28–30 Aside from providing an anatomic basis for classification, it is important to distinguish between chondral and osteochondral lesions, as the natural history and potential for healing varies dramatically between the two entities. It is well recognized that isolated chondral defects have a poor intrinsic capacity for healing, whereas osteochondral defects are able to regenerate a fibrocartilaginous cartilage-like substitute.30–40 The primary impediment toward the healing of chondral lesions is the inherently poor vascularity of articular cartilage and the inability to recruit mesenchymal precursors into the defect, which can create this fibrocartilaginous repair tissue. Articular defects that penetrate through the highly vascularized subchondral bone elicit an aggressive healing response with creation of a hematoma, fibrin clot formation, and influx of inflammatory cytokines and mesenchymal precursors into the lesion. Of course, the repair tissue, which is “hyaline-like” at best and rich in type I collagen, has inferior wear characteristics and durability when compared with native articular hyaline cartilage.41
An effective classification system is characterized by simplicity, interobserver reliability, and the ability to delineate prognosis and guide clinical decision making. Although several classification systems exist for isolated chondral lesions and osteochondritis dissecans (OCD), the literature interestingly lacks a classification system devoted to acute osteochondral lesions.
The most commonly used operative classification system for isolated cartilage lesions continues to be the Outerbridge system, first presented in 1961.42 The Outerbridge, International Cartilage Repair Society (ICRS), and Noyes and Stabler scales describe articular cartilage changes, with the last stage including exposed bone or an osteochondral defect.43,44 Criticisms of these classification systems include a lack of detailed discussion on lesion depth or diameter, articular surface appearance, and location.
In 1959 Berndt and Harty45 reviewed 24 cases and presented a classification system for OCD of the talus that continues to be used based on radiographic findings: stage 1, a small compression fracture, with some authors restricting the size of the fracture to between 1 and 3 cm in diameter; stage 2, an incomplete avulsion of an osteochondral fragment; stage 3, complete avulsion of an osteochondral fragment without displacement; and stage 4, an avulsed fragment and loose body within the joint. This classification system has been extrapolated for use for OCD of the knee.
The classification of osteochondral lesions using magnetic resonance imaging (MRI) is gaining popularity as improvements in imaging techniques have greatly enhanced the sensitivity and specificity of this diagnostic modality. Bohndorf46 reported on a new MRI classification system composed of two distinct stages: stage 1, intact cartilage, contrast enhancement of the lesion, and no cystic defects; stage 2, a cartilage defect with or without incomplete separation of the fragment, fluid around an undetached fragment, and a dislodged fragment.
Osteochondral lesions have traditionally been characterized based on classification schemes applied to OCD lesions, which differ significantly in etiology, epidemiology, clinical presentation, and response to treatment. We propose that acute osteochondral lesions be described based on the following parameters at the time of arthroscopy: (1) location (patella, femur, tibia, weight bearing versus non—weight bearing versus meniscal-bearing regions); (2) size; (3) amount of attached bone (partial chondral, at junction of cartilage to subchondral bone, through subchondral bone into trabecular bone); (4) amount of articular cartilage attached to bone (0–25%, 26–50%, 51–75%, >75%); (5) nondisplaced versus displaced versus free-body; (6) well-fitting versus size or contour mismatch; and (7) integrity of adjacent cartilage in the zone of injury.
 Diagnosis
Diagnosis
Although the presentation of patients with acute osteochondral injury is variable, most complain of pain, joint effusions, and mechanical symptoms. The elicitation of a detailed patient history usually reveals a single traumatic antecedent event associated with the onset of symptoms. This can consist of ligamentous or meniscal injury, blunt contusion, axial loading, or a pivoting event. Pain is by far the most common clinical complaint, and frequently it limits weight bearing. Joint effusions due to hemarthrosis or the influx of inflammatory mediators are quite common and can result in restricted range of motion. Patients with subacute osteochondral fractures may complain of mechanical symptoms such as clicking, popping, and locking, which may be superimposed on an underlying meniscal tear or ligamentous injury.
Physical examination should be done in a systematic manner, with careful observation of gross morphology, the presence of an effusion, and alignment, and it should include palpation of structures, assessment of range of motion, stability testing, and the use of specialized provocative tests. Tenderness at the joint line may be associated with far anterior lesions of the femoral condyles or may occur in the context of underlying meniscal pathology, whereas focal femoral condylar tenderness or a palpable defect more often indicates the presence of an osteochondral defect. Osteochondral lesions secondary to patellar dislocation can result following avulsion of the medial patellofemoral ligament or due to shear and impaction forces with reduction. This subset of patients displays focal tenderness along the medial retinaculum. Following lateral patellar dislocation, up to 50% of patients show evidence of osteochondral lesions of the lateral femoral condyle, medial patellar facet, or both.47 Concomitant ligamentous laxity must be diligently assessed. For the most part, however, the diagnosis is made based on history, advanced imaging studies, and arthroscopic evaluation, as plain radiographs may not always reveal the underlying fragment.
Plain radiographs can be helpful in the diagnosis of osteochondral lesions. A standard trauma series including anteroposterior (AP), lateral, oblique, and Merchant views should be obtained. These views enable the clinician to assess the presence or absence of fractures as well as joint space congruity and narrowing, malalignment, and OCD. However, the amount of bone associated with an osteochondral injury is often small and not detected through the use of routine plain radiographs.
Continuing improvements in MRI enhance its ability to detect articular cartilage pathology and osteochondral injury.48 Proton-density imaging of thin (3–4 mm) sections, T1-weighted fat-suppressed three-dimensional (3D) gradient echo, and T2-weighted fast spin-echo sequences optimize resolution of the articular cartilage and underlying subchondral bone.49,50 The osteochondral fracture may parallel the joint surface or it may present as a periarticular osseous fracture that extends and crosses perpendicular to the articular cartilage. High signal intensity is frequently identified underlying the fracture segment in acute injuries, whereas intermediate intensity signal in cartilage or fibrous tissue is more characteristic of chronic lesions. Absence of hyperintensity of the fragment in the junctional zone is associated with fracture stability.50 High signal intensity fluid surrounding the fragment indicates instability and loosening. MRI is also especially useful in detecting osteochondral loose bodies and chondral fragments. MRI continues to be a highly effective diagnostic tool for the assessment of ligamentous and meniscal pathology as its role in the diagnosis of osteochondral injury continues to evolve.51,52
Compaction injuries or bone bruises represent subchondral microtrabecular fractures and can usually only be diagnosed by MRI scan. These injuries can occur in isolation or more commonly they are seen in association with either an ACL tear or a medial collateral ligament tear. As the knee subluxes due to an ACL tear, impaction occurs between the posterior aspect of the lateral tibial plateau and the sulcus terminalis of the lateral femoral condyle, and increased signal is typically noted on the T2 image at these areas. Johnson et al53 reported that these injuries occur in 80% of complete ACL tears. They described these lesions as being either reticular (limited to the medullary bone) or geographic (extending to the articular surface).53
Computed tomography (CT) can be a useful adjunct to the other imaging methods described earlier, especially in defining the degree of bony involvement. CT is able to demonstrate excellent definition of bony fragments and detailed delineation of size, location, and degree of displacement of the fragment. CT, however, is less sensitive than MRI in defining subtle subchondral microfractures and is limited in the assessment of articular cartilage integrity.
Diagnostic arthroscopy continues to be the gold standard in the assessment, evaluation, and characterization of osteochondral lesions. However, accurate assessment of size may be difficult arthroscopically.
 Surgical Treatment
Surgical Treatment
The ultimate goal in the treatment of osteochondral defects is the restoration of a smooth surface of normal hyaline cartilage bound to a restored subchondral plate and integrated with the adjacent native articular cartilage. This goal has eluded orthopaedic surgeons to this day, yet the current treatment options aim to reduce pain and swelling and improve function. The options currently available to orthopaedic surgeons for the treatment of osteochondral defects can be divided into three broad categories: (1) resection, (2) repair, and (3) replacement.
Resection
The surgical treatment of osteochondral lesions began historically with simple joint debridement. Magnuson54 first coined the term debridement in 1941 for the treatment of osteoarthritis of the knee, which included removal of osteophytes, loose bodies, pathologic cartilage, and hypertrophic synovial tissue. With the advent of arthroscopy and improved techniques and instruments, arthroscopic debridement has evolved considerably in the diagnosis and treatment of osteochondral injury. Although the goals of arthroscopic debridement have remained constant over time (e.g., to reduce pain, improve function, and delay the progression of osteoarthritis), the surgical indications have been variable and continue to generate considerable controversy.55 The lack of prospective, randomized, double-blinded studies examining the role of arthroscopic debridement in the treatment of osteochondral injuries make it difficult to establish well-defined criteria for surgery and for assessing efficacy.
Arthroscopic debridement enables the clinician to systematically examine the knee in detail. Irrigation with lactated Ringer’s solution or normal saline is believed to effectively dilute the synovial fluid of proteolytic and other degradative enzymes, inflammatory mediators, and loose bodies that may contribute to third body wear. Excision of damaged portions of articular cartilage may decrease mechanical symptoms and reduce the release of free particles that can irritate synovial tissue and stimulate inflammation and pain. The pain associated with osteochondral lesions is also attributed to the free nerve endings found in the subchondral bone, which may be sensitive to mechanical irritation by unstable chondral flaps and loose bodies as well as circulating inflammatory mediators.
The decision to perform simple debridement as opposed to repair or replacement is based on a variety of factors such as lesion size, quality of the osteochondral fragment and defect, and patient-specific factors. In general, resection is suitable for osteochondral lesions that (1) are <1 cm in diameter; (2) have comminuted cartilage with or without subchondral bone; and (3) occur in patients who are noncompliant, unmotivated, or otherwise unable to adhere to stringent postoperative rehabilitation protocols. Relative contraindications include (1) age >50, (2) smokers, (3) instability not amenable to reconstruction or repair, (4) intact cartilage with no subchondral bone, and (5) nonambulatory patient.
Debridement can be performed by mechanical methods using a variety of devices such as rotatory shavers and resectors. Debridement to a “stable rim” is a poorly defined term, yet objective data indicate that vertical edges are mechanically advantageous relative to tapered chondral edges.56 Of note, investigators have shown that a visible osteochondral defect averages approximately one-third the final size of the lesion following debridement.29,57 The benefits of arthroscopic debridement include a relatively benign postoperative rehabilitation protocol, with unrestricted weight bearing and range of motion immediately following surgery.
Osteochondral resection can be facilitated by the following technical considerations: Removal of a loose body may be aided by placing the suction on medium-low on the arthroscope or turning the inflow off, and capturing the fragment such that the smallest diameter is longitudinal to the axis of the grasping device. In addition, it is important to make sure that the portal is large enough to enable extraction of the fragment prior to attempting the maneuver. Loose particles are often found in the posteromedial or posterolateral compartments of the knee. They are best visualized by placing the arthroscope through the intercondylar notch between the ACL and lateral femoral condyle when the arthroscope is in the anteromedial portal, and between the posterior cruciate ligament (PCL) and medial femoral condyle when the arthroscope is in the anterolateral portal. An accessory posteromedial or posterolateral portal is often necessary for loose body removal. Finally, when debriding the borders of the osteochondral defect, use a hooded shaver to limit overaggressive debridement.
Repair
Internal fixation is usually indicated for larger osteochondral fragments (≥1 cm diameter) with minimal damage to the articular cartilage. A variety of methods are available, all of which have as a goal anatomic and stable restoration of the articular surface with minimal preparation of the donor and recipient tissue. To optimize articular congruity and wear kinematics, the appropriate height must be reestablished following fixation of the osteochondral fragment. If necessary, iliac crest or proximal tibial autograft or allograft can be placed into the bed of the defect to achieve the appropriate height of the fragment.58
The surgeon has in his arsenal a variety of implants to facilitate stable fixation of the osteochondral fragment including Kirschner wires (K-wires), standard and cannulated AO screws, and lower profile headless, variable-pitch compression screws and bone pegs. In addition, bioabsorbable implants consisting of polylactic acid (PLA), polyglycolic acid (PGA), mixed PLA and PGA, and poly-p-dioxanone (PDS) polymers in the form of pins, screws, and tacks are gaining popularity in the fixation of osteochondral defects.
Fixation of osteochondral fragments began historically with the use of 0.062-inch K-wires placed through the fragment and into subchondral bone.59–61 Rotational stability can be achieved with the use of multiple K-wires placed in tandem; however, wire subsidence can occur. In addition, this technique does not enable compression at the fracture site. To prevent migration and achieve more stable fixation, Hughston et al62 advocated bending the K-wires 1 mm from the tip and seating the wire into the articular cartilage and subchondral bone.
Standard AO screws (small or minifragment) are commonly used, but must be countersunk into the articular cartilage and require a second surgery for hardware removal (Fig. 18–1). The head of the screw may continue to be prominent and damage the opposing articular cartilage, effectively making a unipolar into a bipolar chondral lesion. The Acutrak (Acumed, Beaverton, OR), Herbert (Zimmer, Warsaw, IN), and Heune (Special Devices, Grass Valley, CA) screws are low-profile, headless screws that achieve compression at the fracture site by variable pitch construction, yet are not easily removed and have as a drawback retention of exposed metal in the joint.
Bioabsorbable implants are gaining increased popularity due to progressive load transfer at the osteochondral fracture site as well as the elimination of a required second surgery for hardware removal. PGA polymers, which are the most durable, are characterized by a high rate of resorption. Unfortunately, reports of adverse reactions including late aseptic inflammatory reactions and persistent pain have been found in 6 to 22% of patients.63,64 A lower incidence of these side effects has been found in the PLA, composite, and PDS polymers, given their slower resorptability. The OrthoSorb (Depuy Ace, Warsaw, IN) device carries a resorption profile in between PGA and PLA polymers and is available in 1.3- and 2.0-mm-diameter sizes, but does not allow compression at the fracture site. The SmartNail (Bionx, Tampere, Finland) is a bioabsorbable device composed of a proprietary polymer that is able to function as a compression device in appropriate osteochondral defects (Fig. 18–2).
Indications for repair of an osteochondral defect include (1) lesion size ≥1 cm diameter, (2) fragment with intact articular cartilage with subchondral bone, and (3) compliant patient able to adhere to strict postoperative rehabilitation protocols and weight-bearing restrictions. Relative contraindications include (1) smokers, (2) age >50, and (3) size mismatch of the defect and fragment. Absolute contraindications include (1) uncorrectable ligamentous instability or malalignment, (2) fragment with damaged or comminuted articular cartilage, and (3) noncompliant patients.
Repair may be facilitated by the use of accessory portals as needed, including transpatellar tendon portals to allow correct alignment for placement of pins, screws, and K-wires. Meticulous anatomic reduction as well as compression of the osteochondral fragment must be achieved. In the subacute setting, the subchondral bed should be prepared in an appropriate manner to stimulate bleeding prior to repair of the osteochondral fragment. Provisional fixation using K-wires may be placed into the fragment to prevent rotation prior to placement of the screw. Finally, the surgeon should have a low threshold to convert from an arthroscopic to open procedure if anatomic reduction cannot be obtained.
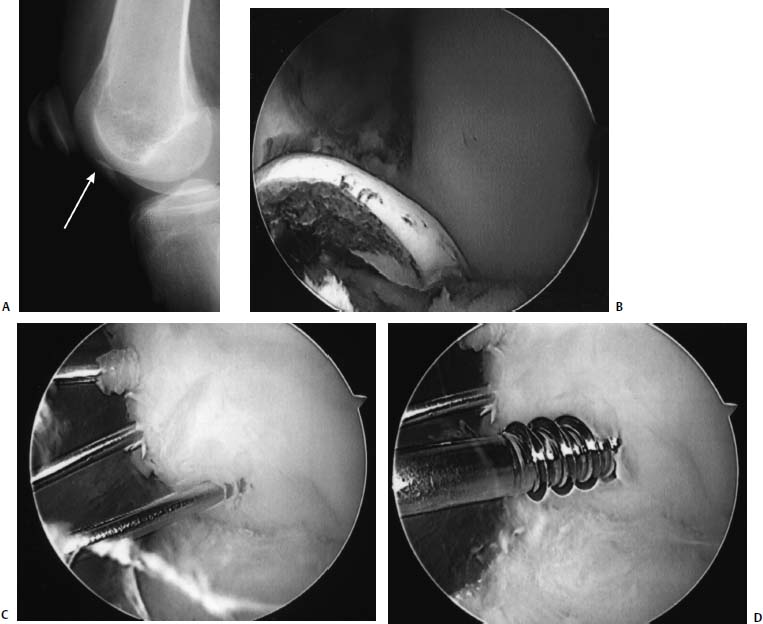
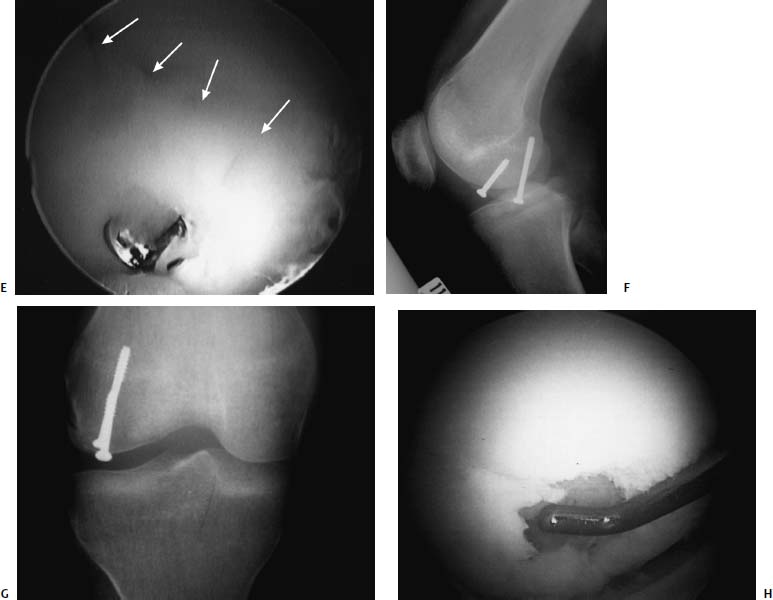
FIGURE 18–1 (A) A 16-year-old high-level ballet dancer sustained an injury pivoting during dance. She noted immediate pain and swelling and was brought to the emergency room, where a bony fragment and effusion could be seen within an otherwise normal radiograph. Examination was suggestive of a reduced patellar dislocation with a fracture from the lateral femoral condyle. Lateral radiograph revealed a loose body (arrow pointing to loose body, seen as a double shadow at the notch). (B) Arthroscopy revealed the osteochondral fracture involving a significant portion of the meniscal bearing area of the lateral femoral condyle. The osteochondral fragment had cancellous bone on the lower half of the fragment, which was approximately 12 mm wide, and only cartilage on the upper half, which was only 6 mm wide. (C) The bony bed was drilled to help stimulate healing. The fragment was then reduced within the defect and held with guidewires for the cannulated 3.5-mm lag screws and for the bioabsorbable pins. (D) The wires for the bioabsorbable pins were maintained to help prevent fragment rotation while drilling and inserting the cannulated screws. The screws were placed first to prevent unnecessary forces on the bioabsorbable pins. (E) Arthroscopic view of the lower half of the reduced fragment with a cannulated screw countersunk is outlined with arrows. Note the anatomic reduction and articular surface congruity of the superomedial edge of the reduced fragment. Arrows point to the edge of the reduced fragment. Lateral (F) and anteroposterior (AP) (G) radiographs 6 weeks following arthroscopic assisted internal fixation of the acute osteochondral fracture. However, also note the healing of the entire lesion, including the upper half, where nearly no bone remained on the fragment. At 2-year follow-up the patient is asymptomatic, has returned to high-level ballet dancing, and full activities without restriction. Her knee examination is normal. Radiographs reveal maintenance of joint space.
The authors’ preferred method of treatment for repairable osteochondral defects is use of a countersunk AO screw with an OrthoSorb pin (Depuy Ace, Warsaw, IN) or SmartNail (Bionx, Tampere, Finland). Patients are placed into immediate continuous passive motion and given non-weight-bearing restrictions for 6 to 8 weeks. Hardware removal and assessment of healing is performed with second-look arthroscopy at 6 to 8 weeks postoperatively. The absorbable devices are used to help provide rotational control of the fragment.
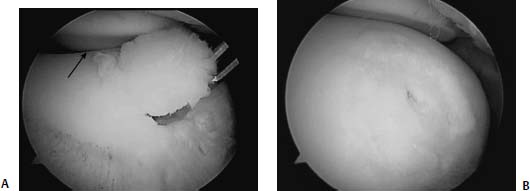
FIGURE 18–2 (A) A 16-year-old boy with an osteochondral injury to the trochlear region of his knee. The lesion is readily identifiable arthroscopically. (B) The bed was exposed arthroscopically to allow for debridement and drilling but maintaining the attachment, like a trapdoor. This lesion was then fixed with an absorbable tack that allows for some compression without the need for removal. Care is taken to ensure that the head of this tack is countersunk to avoid injury to the opposing articular surface.
Osteochondral tibial eminence avulsion fractures usually occur in skeletally immature athletes. These fractures can often include a significant portion of the medial or lateral tibial plateau. They can be reduced arthroscopically and fixed with either cannulated screws or sutures. Often the anterior horn of the lateral meniscus or the intermeniscal ligament blocks the reduction and needs to be retracted prior to reducing the fragment. The ACL tibial guide is often useful for holding the reduction. Using an accessory portal just off the medial or lateral border of the patella, a guidewire can be inserted and a screw placed. An image intensifier should be used and care must be taken not to cross the physes with the screws in a skeletally immature patient (Fig. 18–3). If the bone fragment is too small for screw fixation, sutures may be arthroscopically placed through the ligament stump and brought out through drill holes through the fragment bed and tied over the anteromedial tibia. Cannulated screw fixation has the advantage of facilitating early range of motion. Arthroscopically assisted reduction and cannulated screw fixation is technically much more difficult in tibial eminence avulsions in adults, and usually mini-open reduction and fixation is preferred for adults.
Osteochondral fractures of the patella are usually a result of a patellar dislocation and often involve the medial facet of the patella. This type of fracture is often not amenable to arthroscopic fixation. If the fragment is reparable, an arthrotomy can be made and the osteochondral fragment fixed using screws or bioabsorbable pins as previously described. Suture fixation has also been described for this type of fracture.65 Keith needles can be drilled from the articular surface through the reduced fragment and brought out the anterior surface of the patella. The skin and subcutaneous tissue is dissected off the anterior surface of the patella and the needles are identified. Sutures are then passed through the needle eyelets and the needles are brought out the anterior surface of the patella. The sutures can then be tied over the anterior surface of the patella. If four needles are used, then two sutures can be used in a cross pattern, which provides stable fixation for the osteochondral fragment (Fig. 18–4).
Replacement
The replacement of damaged osteochondral articular defects is indicated for those lesions greater than 1 cm in diameter that are not amenable to primary repair. As discussed in detail elsewhere in this book, it can be achieved by two specific strategies: (1) intrinsic replacement, and (2) transplantation. Intrinsic replacement is based on stimulating intrinsic repair mechanisms within the articular cartilage and subchondral bone to regenerate or reconstitute the osteochondral defect. An alternative approach is the transplantation of autologous or allogeneic chondrocytes, chondrogenic cells, or tissue that have the potential to grow into new cartilage and repair the defect.
Intrinsic replacement can be induced by subchondral drilling, abrasion, or microfracture techniques, which differ mainly in their method of achieving subchondral penetration. The theoretical goal is the recruitment of mesenchymal pluripotential cells into the defect by penetrating the subchondral bone, which can reconstitute a hyaline-like repair tissue.
Subchondral drilling was first reported by Smillie61 in the open treatment of an osteochondral fragment in 1957. Several investigators have subsequently advocated subchondral drilling in isolation or in conjunction with fixation or other replacement procedures.56,60–62,66–69 This technique poses the risk of thermal necrosis to articular cartilage and subchondral bone and has been supplanted by newer methods such as abrasion chondroplasty or microfracture.
Abrasion chondroplasty was pioneered by Johnson70 and is a variation in concept of the subchondral drilling technique. Abrasion chondroplasty involves abrasion of the articular sclerotic defect with an arthroscopic burr to enable bleeding into the articular defect and promote a fibrocartilaginous repair tissue to reconstitute the lesion; however, this was proposed as a treatment for chondral injury with intact subchondral plate. A study has indicated that the integrity of this repair tissue can be maintained for up to 6 years postoperatively.70 Other studies have reported less favorable outcomes with a rapid deterioration of improvement over time.71,72 Although this technique has been advocated for chondral lesions with intact subchondral bone, the concept has been extrapolated to the treatment of osteochondral lesions; however, scientific confirmation is lacking.
The microfracture technique, popularized by Steadman, is performed by creating multiple small holes into the subchondral bone using specialized awls that results in “microfracture” of the trabeculae rather than destruction of bone.73,74 The lesion is debrided and subchondral bone exposed. The arthroscopic awls are used to make microfractures by picking three or four holes per square centimeter to a depth of ~4 mm. Heat necrosis is avoided and the integrity of the subchondral bone shape is maintained, whereas the roughened subchondral surface allows better adherence of the clot. Continuous passive motion and no weight bearing for 6 to 8 weeks is essential for fibrocartilaginous tissue maturation, adherence, and pain relief. Steadman et al74 reported a 75% improvement at 3- to 5-year follow-up using this technique. Again, while this technique has been extensively investigated in the treatment of isolated chondral lesions, the concept may be applied in the setting of subchondral bone loss.
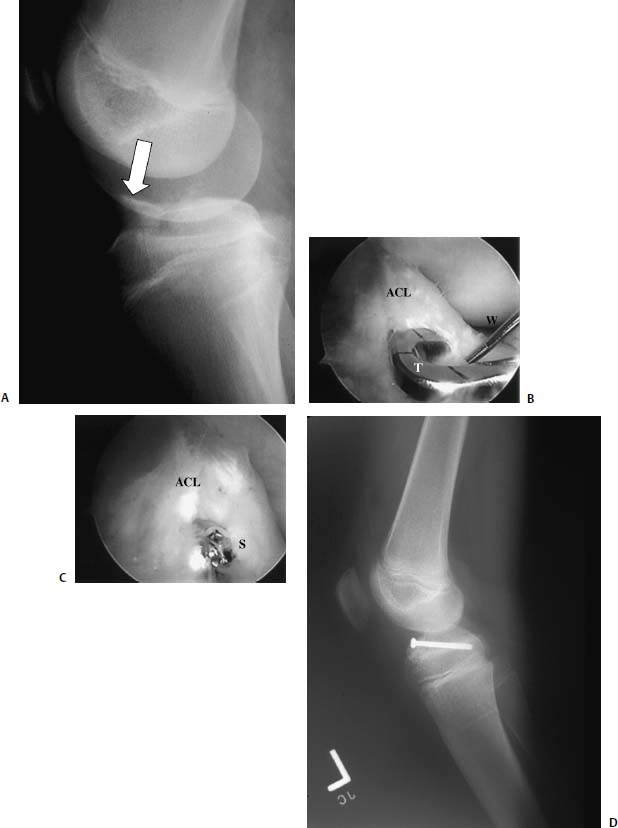
FIGURE 18–3 (A) A 12-year-old boy with a type II tibial eminence avulsion fracture sustained while skateboarding. The fracture fragment (tibial eminence) is outlined by solid straight arrow. (B,C) The anterior cruciate ligament (ACL) tibial guide (T) is helpful in holding the reduction while the guidewire (W) is inserted. A 4.0-cannulated screw (S) is the inserted over the guidewire. (D) Postoperative lateral radiograph. Care must be taken to keep the screw above the physis in a skeletally immature patient.
Transplantation of osteochondral autograft was first reported by Judet and Henri75 in 1908 for the treatment of OCD. The osteochondral autograft transplantation system (OATS) (Arthrex, Naples, FL) procedure involves harvest and implantation of a single osteochondral cylinder, whereas mosaicplasty (Acufex, Mansfield, MA), popularized by Hangody in 1994, involves the harvest and implantation of multiple osteochondral cylinders that are press-fit into drill holes.76 Mosaicplasty has been recommended for much larger and deeper osteochondral lesions (1 to 8 cm2). The donor site is usually a relatively non-weight-bearing region of the knee such as the edge of the patellar groove or the area just proximal to the intercondylar notch.
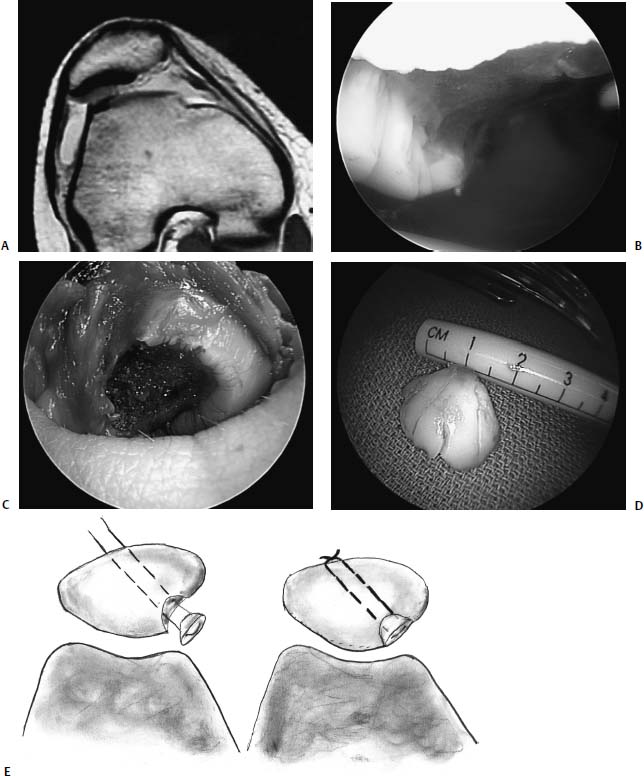
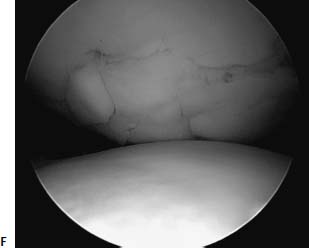
FIGURE 18–4 (A) Medial facet patella (arrow) fracture seen on MRI after patella dislocation. (B) Arthroscopic view of patellar defect. (C) An arthrotomy is usually necessary when fixing an osteochondral fracture of the patella. (D) Fracture fragment is measured on the back table. (E) Keith needles can be used to hold the fracture reduced. The needles are then used to pass poly-p-dioxanone (PDS) sutures that can be tied to secure the fragment. (F) Medial facet patella osteochondral fracture reduction using suture fixation. (Photo courtesy of Raffy Mirzayan, M.D.)
A key component of considering this line of treatment is the necessity for normal knee biomechanics and stability. If abnormal knee alignment or instability is present, it requires simultaneous treatment at the time of osteochondral implantation. Conventional instrumentation systems facilitate arthroscopic osteochondral autografting for limited lesions of the femoral condyles, whereas open arthrotomy is required for lesions of the patella, trochlea, and posterior femoral condyles. Many surgeons find it easier to place the graft perpendicular to the defect using an open or miniarthrotomy approach. Complications of the two procedures include hemarthrosis, effusion, donor-site pain, graft fracture, and osteochondral loose bodies, which can exacerbate the above symptoms.
Osetochondral allografts were first reported in 1925 with the work of Lexer,77 who reported on osteochondral transplantation of articular cartilage in knees, fingers, and elbows. As increased understanding of the immune system emerged, so did a greater interest in transplantation techniques in the 1960s. Osteochondral allografts may be used for larger osteochondral lesions that are not candidates for autologous replacement or have failed prior restorative efforts. This technique is attractive due to the lack of donor-site morbidity, but it carries the risk of disease transmission, graft settling, poor incorporation, and difficulty with availability. Fresh allografts, obtained within 72 hours, carry the greatest chance of chondrocyte survival and likelihood of integration, yet they also carry the risk of higher disease transmission and immunogenicity. The use of shell allografts, or allografts with less than 1 cm of subchondral bone, reduces the chance of disease transmission by decreasing exposure to leukocytes found in cancellous bone. The preservation of osteochondral allografts is an area of ongoing research, but currently fresh grafts can be preserved to near 100% viability for up to 4 days at 4°C. Fresh grafts are stored for up to 21 days following appropriate harvesting. Frozen grafts are also available, but they show an increased prevalence of fissuring, delamination, fibrosis, and generalized breakdown of the articular surface, making them less attractive for implantation.
The success of osteochondral allografts is dependent on the viability and fixation of the subchondral bone of the graft to native bone. Despite improvements in immunosuppressive techniques, “creeping substitution” is a major impediment to allograft viability as the host immune system can initiate significant tissue remodeling of the allograft. The speed of this process is reduced by “cork” fixation of the allograft, where the graft is shaped like a tapered cone and press-fitted into the defect. Creeping substitution, however, is increased when fixation penetrates the entire graft, with either permanent or bioabsorbable implants.78
In autologous chondrocyte implantation (ACI), chondrocytes are harvested arthroscopically, expanded in culture, and transplanted into the defect beneath a periosteal flap 2 to 5 weeks thereafter. This technique was initially described by Brittberg et al79 for the treatment of symptomatic full-thickness chondral injuries in select patients. The technique has also been advocated in the treatment of osteochondral lesions in OCD, with Peterson et al80 and Minas and Nehrer81 recommending bone grafting for lesions greater than 8 mm in depth. Little data exists as to the efficacy of ACI in the treatment of acute osteochondral defects of the knee.
Rib perichondrium has been used as an autologous graft source in the treatment of osteochondral defects in young patients, but it has fallen out of favor due to the high incidence of calcification of the perichondrium.
Technical impediments characteristic of all replacement techniques include difficulty with perpendicular graft placement, graft impaction forces, cartilage thickness and stiffness mismatch, and radius of curvature differences.
The use of periosteum in the treatment of osteochondral lesions is based on the ability of the cambium layer to differentiate into chondrocytes and form hyaline cartilage.7 Prior investigators have shown that when periosteal tissue is sutured into the base of an osteochondral lesion, a hyaline-like repair tissue (87% type II collagen) is generated with similar biomechanical characteristics of hyaline cartilage. Success of this technique is augmented with the use of continuous passive motion postoperatively, and this has been confirmed in animal studies.82 This technique has been successful in the treatment of lesions of the patella and femoral condyle as well as diminishing pain in select patients. There continues to be controversy regarding the orientation of the cambium layer into the defect.
A restorative procedure is indicated for those lesions larger than 1 cm in diameter that do not meet the criteria for repair due to the quality of the articular cartilage of the fragment, lack of suitable subchondral bone attached to the fragment, or a lack of suitable implants. Contraindications continue to include smokers and noncompliant patients. In addition, given the absence of any scientific data confirming the benefit of one technique over another, the preferred intervention should be based on surgeon technical experience and comfort. Osteochondral lesions less than 2.5 cm in diameter may be repaired with multiple 1-cm autologous osteochondral grafts obtained from a non—weight-bearing portion of the knee with approximately two-thirds the height of the plug buried within the subchondral bone at the base of the defect. The authors’ preference for lesions greater than 2.5 cm in diameter is to use fresh allograft tissue or autologous chondrocyte implantation.
 Postoperative Care
Postoperative Care
Continuous Passive Motion
Salter83,84 introduced the use of continuous passive motion (CPM) of knees for the postoperative treatment of full-thickness articular cartilage lesions as well as intraarticular fractures. In one of Salter’s studies, four 1-mm drill holes were created in rabbit knees; hyaline cartilage healing occurred in 60% of rabbits receiving CPM as compared with 10% in those that were immobilized or allowed unrestricted cage activity.83 Although subsequent studies have shown that CPM is less effective in defects larger than 3 mm in diameter, it is generally regarded to be adjunctive in any procedure directed toward chondral and osteochondral healing.85–87
 Complications
Complications
Apoptosis and Osteochondral Injury
Studies have shown that chondrocyte apoptosis, or programmed cell death (PCD), is associated with osteochondral injury and may lead to the development of posttraumatic arthritis.15,88–93 Experimental cartilage injuries such as compression, drilling, and trephine wounding can stimulate significantly increased levels of chondrocyte apoptosis in vitro.94 Even the presence of a hemarthrosis is deleterious to chondrocyte viability and stimulates apoptosis.6 Given the poor intrinsic regenerative capacity of hyaline articular cartilage, there has been increasing interest in the possibility of preserving chondrocytes following injury by inhibiting key enzymes in the apoptotic cascade.95
Commonly performed arthroscopic procedures such as chondral shaving, debridement, and laser abrasion lead to death of chondrocytes extending beyond the border of the treatment site and may have a deleterious effect on articular cartilage long-term.96,97 In a recent study, Hunziker and Driesang98 reported significant chondrocyte loss at the wound edge of partial-thickness chondral defects in pigs repaired with an autologous fascial flap sutured over a fibrinous matrix. Even seemingly benign procedures such as the suturing of a periosteal flap during ACI may result in chondrocyte death along the path of the suture needle. Thus, the iatrogenic creation of an acellular or hypocellular zone of cartilage may be a major factor affecting edge healing and incorporation of transplanted repair tissue. Future research is needed to determine whether apoptosis inhibition may be a useful adjunct in the treatment of acute osteochondral injury by limiting chondrocyte death following trauma and in improving the results of current cartilage repair and replacement techniques.
 Conclusion
Conclusion
Not enough studies have been done yet to provide the scientific validation needed to guide the treatment of acute osteochondral defects of the knee. Basic tenets of articular fracture management prevail and guide the current treatment algorithms, emphasizing anatomic and stable reduction of the fragment, establishment of articular surface congruity, and the use of compressive techniques to facilitate fracture healing. Early range of motion, the use of continuous passive motion, and accelerated rehabilitation protocols are beneficial for articular cartilage viability and have been shown to accelerate healing. Future developments in stimulating bone and cartilage healing on the cellular level, in limiting programmed cell death following injury and iatrogenic preparation of the defect, and in implant options will likely enable the orthopaedic surgeon to improve surgical outcomes.
REFERENCES
1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy 1997;13:456–460
2. Messner K, Maletius W. The long-term prognosis for severe damage to weight-bearing cartilage in the knee: a 14-year clinical and radiographic follow-up in 28 young athletes. Acta Orthop Scand 1996;67:165–168
3. Blanco FJ, Ochs RL, Schwarz H, Lotz M. Chondrocyte apoptosis induced by nitric oxide. Am J Pathol 1995;146:75–85
4. Frenkel J, Sherman D, Fein A, et al. Accentuated apoptosis in normally developing p53 knockout mouse embryos following genotoxic stress. Oncogene 1999;18:2901–2907
5. Hashimoto S, Takahashi K, Amiel D, Coutts RD, Lotz M. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis Rheum 1998;41:1266–1274
6. Hooiveld M, Roosendaal G, Wenting M, van den Berg M, Bijlsma J, Lafeber F. Short-term exposure of cartilage to blood results in chondrocyte apoptosis. Am J Pathol 2003;162:943–951
7. Buckwalter JA, Mow VC, Ratcliffe A. Restoration of injured or degenerated articular cartilage. J Am Acad Orthop Surg 1994;2: 192–201
8. Buckwalter JA, Martin JA, Olmstead M, Athanasiou KA, Rosenwasser MP, Mow VC. Osteochondral repair of primate knee femoral and patellar articular surfaces: implications for preventing post-traumatic osteoarthritis. Iowa Orthop J 2003;23:66–74
9. Zhu W, Mow VC, Koob TJ, Eyre DR. Viscoelastic shear properties of articular cartilage and the effects of glycosidase treatments. J Orthop Res 1993;11:771–781
10. Donohue JM, Buss D, Oegema TR Jr, Thompson RC Jr. The effects of indirect blunt trauma on adult canine articular cartilage. J Bone Joint Surg Am 1983;65:948–957
11. Radin EL, Paul IL. Response of joints to impact loading. I. In vitro wear. Arthritis Rheum 1971;14:356–362
12. Radin EL, Parker HG, Pugh JW, Steinberg RS, Paul IL, Rose RM. Response of joints to impact loading. 3. Relationship between trabecular microfractures and cartilage degeneration. J Biomech 1973;6:51–57
13. Repo RU, Finlay JB. Survival of articular cartilage after controlled impact. J Bone Joint Surg Am 1977;59:1068–1076
14. Vener MJ, Thompson RC Jr, Lewis JL, Oegema TR Jr. Subchondral damage after acute transarticular loading: an in vitro model of joint injury. J Orthop Res 1992;10:759–765
15. D’Lima DD, Hashimoto S, Chen PC, Colwell CW Jr, Lotz MK. Impact of mechanical trauma on matrix and cells. Clin Orthop 2001;391(suppl):S90–99
16. Tomatsu T, Imai N, Takeuchi N, Takahashi K, Kimura N. Experimentally produced fractures of articular cartilage and bone. The effects of shear forces on the pig knee. J Bone Joint Surg Br 1992;74:457–462
17. Li X, Haut RC, Altiero NJ. An analytical model to study blunt impact response of the rabbit P-F joint. J Biomech Eng 1995;117: 485–491
18. Borrelli J Jr, Torzilli PA, Grigiene R, Helfet DL. Effect of impact load on articular cartilage: development of an intra-articular fracture model. J Orthop Trauma 1997;11:319–326
19. Simon SR, American Academy of Orthopaedic Surgeons. Orthopaedic Basic Science. Rosemont, IL: American Academy of Orthopaedic Surgeons, 1994
20. Setton LA, Mow VC, Howell DS. Mechanical behavior of articular cartilage in shear is altered by transection of the anterior cruciate ligament. J Orthop Res 1995;13:473–482
21. Moskowitz RW, Davis W, Sammarco J, et al. Experimentally induced degenerative joint lesions following partial meniscectomy in the rabbit. Arthritis Rheum 1973;16:397–405
22. Aizawa T, Kon T, Einhorn TA, Gerstenfeld LC. Induction of apoptosis in chondrocytes by tumor necrosis factor-alpha. J Orthop Res 2001;19:785–796
23. Hashimoto S, Creighton-Achermann L, Takahashi K, Amiel D, Coutts RD, Lotz M. Development and regulation of osteophyte formation during experimental osteoarthritis. Osteoarthritis Cartilage 2002;10:180–187
24. Lucchinetti E, Adams CS, Horton WE Jr, Torzilli PA. Cartilage viability after repetitive loading: a preliminary report. Osteoarthritis Cartilage 2002;10:71–81
25. Kobayashi K, Mishima H, Hashimoto S, et al. Chondrocyte apoptosis and regional differential expression of nitric oxide in the medial meniscus following partial meniscectomy. J Orthop Res 2001;19:802–808
26. Asada S, Fukuda K, Nishisaka F, Matsukawa M, Hamanisi C. Hydrogen peroxide induces apoptosis of chondrocytes; involvement of calcium ion and extracellular signal-regulated protein kinase. Inflamm Res 2001;50:19–23
27. Kempson GE, Muir H, Pollard C, Tuke M. The tensile properties of the cartilage of human femoral condyles related to the content of collagen and glycosaminoglycans. Biochim Biophys Acta 1973;297:456–472
28. Sokolof L. The Biology of Degenerative Joint Disease. Chicago: University of Chicago Press, 1969
29. Terry GC, Flandry F, Van Manen JW, Norwood LA. Isolated chondral fractures of the knee. Clin Orthop 1988;234:170–177
30. Hunter W. Of the structure and disease of articulating cartilages. Clin Orthop 1995;317:3–6
31. Bennett G, Baur W, Maddock SJ. A study of the repair of articular cartilage and the reaction of normal joints of adult dogs to surgically created defects of articular cartilage, “joint mice,” and patellar displacement. Am J Pathol 1932;8:499–524
32. Bennett G, Baur W. Further studies concerning the repair of articular cartilage in dog joints. J Bone Joint Surg Am 1935; 17: 141–150
33. Calandruccio R, Gilmer WS. Proliferation, regeneration, and repair of articular cartilage of immature animals.J Bone Joint Surg Am 1962;44A:431–455
34. Campbell CJ. The healing of cartilage defects. Clin Orthop Relat Res 1969;64:45–63
35. DePalma AF, McKeever CD, Subin DK. Process of repair of articular cartilage demonstrated by histology and autoradiography with tritiated thymidine. Clin Orthop 1966;48:229–242
36. Fuller JA, Ghadially FN. Ultrastructural observations on surgically produced partial-thickness defects in articular cartilage. Clin Orthop 1972;86:193–205
37. Ito L. The nutrition of articular cartilage and its method of repair. Br J Surg 1924;12:31–42
38. Mankin HJ. Localization of tritiated thymidine in articular cartilage of rabbits. II. Repair in immature cartilage. J Bone Joint Surg Am 1962;44A:688–698
39. Mankin HJ. The reaction of articular cartilage to injury and osteoarthritis (second of two parts). N Engl J Med 1974;291: 1335–1340
40. Paget J. Healing of cartilage. Clin Orthop 1969;64:7–8
41. Moskowitz RW. Osteoarthritis, Diagnosis and Medical/Surgical Management, 2nd ed. Philadelphia: Saunders, 1992:71–107
42. Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br 1961;43-B:752–757
43. Noyes FR, Stabler CL. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med 1989;17:505–513
44. Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am 2003;85-A(suppl 2):58–69
45. Berndt AL, Harty M. Transchondral fractures (osteochondritis dissecans) of the talus. Am J Orthop 1959;41-A:988–1020
46. Bohndorf K. Osteochondritis (osteochondrosis) dissecans: a review and new MRI classification. Eur Radiol 1998;8: 103–112
47. Boden BP, Pearsall AW, Garrett WE Jr, Feagin JA Jr. Patellofemoral instability: evaluation and management. J Am Acad Orthop Surg 1997;5:47–57
48. Potter HG. Imaging of posttraumatic and soft tissue dysfunction of the elbow. Clin Orthop 2000;370:9–18
49. Kibler WB, Herring SA, Press JM. Functional Rehabilitation of Sports and Musculoskeletal Injuries. Gaithersburg, MD: Aspen, 1998:20–56
50. Stoller DW. Magnetic Resonance Imaging in Orthopaedics and Sports Medicine, 2nd ed. Philadelphia: Lippincott-Raven, 1997:419
51. Rangger C, Klestil T, Kathrein A, Inderster A, Hamid L. Influence of magnetic resonance imaging on indications for arthroscopy of the knee. Clin Orthop 1996;330:133–142
52. Jackson DW, Jennings LD, Maywood RM, Berger PE. Magnetic resonance imaging of the knee. Am J Sports Med 1988;16:29–38
53. Johnson DL, Urban WP Jr, Caborn DN, Vanarthos WJ, Carlson CS. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med 1998;26:409–414
54. Magnuson PB. The classic: joint debridement: surgical treatment of degenerative arthritis. Clin Orthop 1974;101:4–12
55. Moseley JB, O’Malley K, Petersen NJ, et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002;347:81–88
56. Dzioba RB. The classification and treatment of acute articular cartilage lesions. Arthroscopy 1988;4:72–80
57. Levy AS, Lohnes J, Sculley S, LeCroy M, Garrett W. Chondral delamination of the knee in soccer players. Am J Sports Med 1996;24:634–639
58. Gillespie HS, Day B. Bone peg fixation in the treatment of osteochondritis dissecans of the knee joint. Clin Orthop 1979;143: 125–130
59. Guhl JF. Arthroscopic treatment of osteochondritis dissecans: preliminary report. Orthop Clin North Am 1979;10:671–683
60. Lipscomb PR Jr, Lipscomb PR Sr, Bryan RS. Osteochondritis dissecans of the knee with loose fragments. Treatment by replacement and fixation with readily removed pins. J Bone Joint Surg Am 1978;60:235–240
61. Smillie IS. Treatment of osteochondritis dissecans. J Bone Joint Surg Br 1957;39-B:248–260
62. Hughston JC, Hergenroeder PT, Courtenay BG. Osteochondritis dissecans of the femoral condyles. J Bone Joint Surg Am 1984;66:1340–1348
63. Song EK, Lee KB, Yoon TR. Aseptic synovitis after meniscal repair using the biodegradable meniscus arrow. Arthroscopy 2001;17: 77–80
64. Menche DS, Phillips GI, Pitman MI, Steiner GC. Inflammatory foreign-body reaction to an arthroscopic bioabsorbable meniscal arrow repair. Arthroscopy 1999;15:770–772
65. Dhawan A, Hospodar PP. Suture fixation as a treatment for acute traumatic osteochondral lesions. Arthroscopy 1999;15:307–311
66. Aglietti P, Buzzi R, Bassi PB, Fioriti M. Arthroscopic drilling in juvenile osteochondritis dissecans of the medial femoral condyle. Arthroscopy 1994;10:286–291
67. Anderson AF, Richards DB, Pagnani MJ, Hovis WD. Antegrade drilling for osteochondritis dissecans of the knee. Arthroscopy 1997;13:319–324
68. Bradley J, Dandy DJ. Results of drilling osteochondritis dissecans before skeletal maturity. J Bone Joint Surg Br 1989;71:642–644
69. Rae PJ, Noble J. Arthroscopic drilling of osteochondral lesions of the knee. J Bone Joint Surg Br 1989;71:534
70. Johnson LL. Arthroscopic abrasion arthroplasty historical and pathologic perspective: present status. Arthroscopy 1986;2:54–69
71. Bert JM. Electrocautery. Arthroscopy 1991;7:414–415
72. Bert JM. Role of abrasion arthroplasty and debridement in the management of osteoarthritis of the knee. Rheum Dis Clin North Am 1993;19:725–739
73. Steadman JR, Rodkey WG, Rodrigo JJ. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop 2001;391(suppl):S362–369
74. Steadman JR, Rodkey WG, Briggs KK. Microfracture to treat full-thickness chondral defects: surgical technique, rehabilitation, and outcomes. J Knee Surg 2002;15:170–176
75. Henri JA, Judet. Essai sur la greffe des tissues articulares. Comp Rend Acad D Sci. 1908;146:193–196
76. Hangody L, Kish G, Karpati Z, Udvarhelyi I, Szigeti I, Bely M. Mosaicplasty for the treatment of articular cartilage defects: application in clinical practice. Orthopedics 1998;21:751–756
77. Lexer E. Joint transplantations and arthroplasty. Surg Gynecol Obstet 1925;40:782–809
78. Browne JE, Branch TP. Surgical alternatives for treatment of articular cartilage lesions. J Am Acad Orthop Surg 2000;8:180–189
79. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med 1994;331: 889–895
80. Peterson L, Minas T, Brittberg M, Lindahl A. Treatment of osteochondritis dissecans of the knee with autologous chondrocyte transplantation: results at two to ten years. J Bone Joint Surg Am 2003;85-A(suppl 2):17–24
81. Minas T, Nehrer S. Current concepts in the treatment of articular cartilage defects. Orthopedics 1997;20:525–538
82. Delaney JP, O’Driscoll SW, Salter RB. Neochondrogenesis in free intraarticular periosteal autografts in an immobilized and paralyzed limb. An experimental investigation in the rabbit. Clin Orthop 1989;248:278–282
83. Salter RB, Simmonds DF, Malcolm BW, Rumble EJ, MacMichael D, Clements ND. The biological effect of continuous passive motion on the healing of full-thickness defects in articular cartilage. An experimental investigation in the rabbit. J Bone Joint Surg Am 1980;62:1232–1251
84. Salter RB. Continuous Passive Motion (CPM): A Biological Concept for the Healing and Regeneration of Articular Cartilage, Ligaments, and Tendons: From Its Origination to Research to Clinical Applications. Baltimore: Williams & Wilkins, 1993
85. O’Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am 1986;68:1017–1035
86. O’Driscoll SW, Salter RB. The repair of major osteochondral defects in joint surfaces by neochondrogenesis with autogenous osteoperiosteal grafts stimulated by continuous passive motion. An experimental investigation in the rabbit. Clin Orthop 1986;208: 131–140
87. Rodrigo JJ, Steadman JR, Syftestad G, Benton H, Silliman J. Effects of human knee synovial fluid on chondrogenesis in vitro. Am J Knee Surg 1995;8:124–129
88. Blanco FJ, Guitian R, Vazquez-Martul E, de Toro FJ, Galdo F. Osteoarthritis chondrocytes die by apoptosis. A possible pathway for osteoarthritis pathology. Arthritis Rheum 1998;41: 284–289
89. Hashimoto S, Ochs RL, Komiya S, Lotz M. Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum 1998;41:1632–1638
90. D’Lima DD, Hashimoto S, Chen PC, Lotz MK, Colwell CW Jr. Prevention of chondrocyte apoptosis. J Bone Joint Surg Am 2001;83-A(suppl 2, pt 1):25–26
91. D’Lima DD, Hashimoto S, Chen PC, Colwell CW Jr, Lotz MK. Human chondrocyte apoptosis in response to mechanical injury. Osteoarthritis Cartilage 2001;9:712–719
92. D’Lima DD, Hashimoto S, Chen PC, Lotz MK, Colwell CW Jr. In vitro and in vivo models of cartilage injury. J Bone Joint Surg Am 2001;83-A(suppl 2, pt 1):22–24
93. Kim HT, Lo MY, Pillarisetty R. Chondrocyte apoptosis following intraarticular fracture in humans. Osteoarthritis Cartilage 2002; 10:747–749
94. Tew SR, Kwan AP, Hann A, Thomson BM, Archer CW. The reactions of articular cartilage to experimental wounding: role of apoptosis. Arthritis Rheum 2000;43:215–225
95. Costouros JG, Dang AC, Kim HT. Inhibition of chondrocyte apoptosis in vivo following acute osteochondral injury. Osteoarthritis Cartilage 2003;11:756–759
96. Hunziker EB. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthritis Cartilage 1999;7:15–28
97. Hunziker EB, Quinn TM. Surgical removal of articular cartilage leads to loss of chondrocytes from cartilage bordering the wound edge. J Bone Joint Surg Am 2003;85-A(suppl 2): 85–92
98. Hunziker EB, Driesang IMK. Surgical suturing of adult articular cartilage is associated with a loss of chondrocytes and an absence of wound healing. Transactions of the 49th annual meeting of the Orthopaedic Research Society. 2003.
< div class='tao-gold-member'>



