(1)
Department of Orthopedic Surgery Academic Medical Centre, University of Amsterdam, Amsterdam, The Netherlands
Abstract
This chapter describes endoscopic management of isolated paratendinopathy and endoscopic management of a combination of midportion tendinopathy with paratendinopathy. The approach by means of Achilles tendoscopy is the release of adhesions of the peritendineum, denervation of the tendon, removal of the pathologic tissue of the peritendineum, and endoscopic release or resection of the plantaris tendon at the level of the painful nodule in the Achilles tendon. This chapter describes the anatomical consideration, etiology, and symptoms of Achilles paratendinopathy and tendinopathy. The endoscopic treatment including portals, rehabilitation, and results is given in detail.
Achilles tendinopathy involves pathology of the tendon proper and includes the histopathological diagnosis of tendinosis.
Achilles paratendinopathy is defined by degeneration and/or inflammation of the thin membrane around the Achilles tendon. Exercise-induced pain and local swelling around the tendon’s midportion are the main symptoms.
The current endoscopic approach to isolated or combined tendinopathy and paratendinopathy is the release of adhesions of the peritendineum, denervation of the tendon, removal of the pathologic peritendinous tissue, and endoscopic release of the plantaris tendon.
22.1 Introduction
Degeneration of the body of the Achilles tendon has been found during autopsies in 34 % of tendons in patients without complaints (Kannus and Jozsa 1991). This finding was confirmed in a study by Khan and coworkers who identified abnormal tendon morphology in 37 of the 57 symptomatic tendons (65 %) and abnormal morphology in 9 of 28 asymptomatic tendons (32 %) using ultrasound (Khan et al. 2003). Another study concluded that there was no relationship between symptoms and ultrasonographic intratendinous abnormalities in elite gymnasts. These studies imply that degeneration of the Achilles tendon proper is not the source of pain (Fig. 22.1).
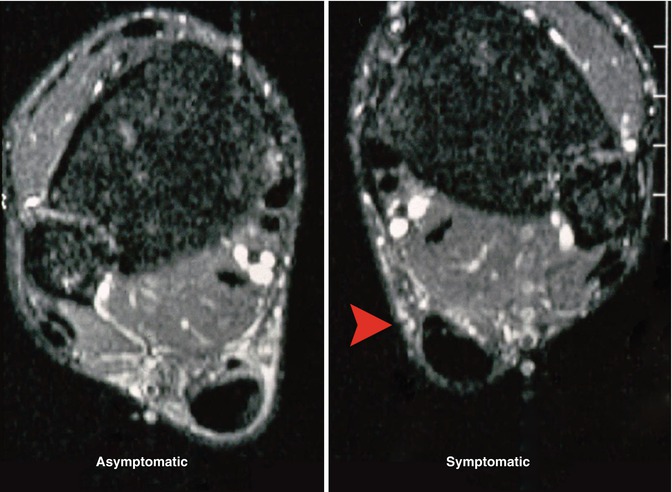

Fig. 22.1
A patient with chronic midportion Achilles tendon pain on the right side. On clinical examination, swelling of the midportion of the Achilles tendon on both sides (nodular thickening). On the left side, no pain and no tenderness on palpation. On the right side, recognizable tenderness on palpation over the nodule, on the medial side more than on the lateral side (red arrow). MRI shows nodular thickening of both tendons with degeneration on the left side (the asymptomatic side!). The plantaris tendon can be seen here as a separate structure. On the symptomatic right ankle, we see the plantaris tendon fixed to the Achilles tendon. Note the dense paratenon. Compare to the uninvolved paratenon on the asymptomatic side: the tendon is surrounded here by radiolucent tissue
Neovascularization, enhanced neurovascular growth into the peritendineum, and myofibroblasts responsible for the formation of permanent scarring and the shrinkage of peritendinous tissue have been shown in patients with chronic Achilles tendinopathy (Van Sterkenburg et al. 2010b). It is my strong belief that the main cause of pain in patients with Achilles tendinopathy comes from the peritendineum and not from the tendon itself.
This chapter describes the endoscopic management of a combination of midportion tendinopathy, paratendinopathy, and isolated paratendinopathy: Achilles tendoscopy.
22.2 Rationale for Treatment
Achilles tendinopathy is a clinical entity that is generally difficult to treat. The source of pain and the background to the pain mechanisms associated with it have not been scientifically clarified (Maffulli et al. 2004). A wide range of conservative and surgical treatments are available. Why surgery promotes healing of the Achilles tendon is still not fully understood (Sandmeier and Renström 1997). Often the intratendinous degenerative changes are addressed. However, it is questionable if degeneration of the tendon itself is the main cause of the pain in tendinopathy (Van Sterkenburg et al. 2010a). Several studies have reported intratendinous changes in cadaver specimens and MRI images in patients without complaints (Emerson et al. 2010; Haims et al. 2000; Kannus and Jozsa 1991; Khan et al. 2003). Recently a long-term follow-up study was published by Alfredson and coworkers revealing persistent structural abnormalities and thickening of the tendon 13 years after intratendinous surgery for Achilles tendinopathy, whereas all patients were satisfied with the results and went back to Achilles tendon loading activities without restrictions (Alfredson et al. 2009).
Achilles tendinopathy and paratendinopathy often coexist (Tan and Chan 2008). In patients with a clinical combination of these entities, the pain is often most prominent on the medial side of the midportion of the Achilles tendon (Segesser et al. 1995), where the plantaris tendon runs closely parallel to the Achilles tendon.
The plantaris tendon originally is a knee and ankle flexor. In primates, it attaches to the plantar aspect of the proximal interphalangeal joints of the toes explaining its functionality for grasping with their feet. Darwinists suggest that through evolution in humans the plantaris tendon is rudimental, since we do not grasp with our feet nor swing from trees any longer. Absence in 7–20 % of human lower limbs has been reported (Danforth 1923; Daseler 1943; Freeman et al. 2008; Moss 1988; Schwalbe and Pfitzner 1894).
In humans, the plantaris muscle is triangularly shaped and lies posterior to the knee joint, originating from the inferior part of the lateral supracondylar line of the femur (Figs. 22.2 and 22.3). Its tendon travels inferomedially, posterior to the soleus muscle and anterior to the medial gastrocnemius muscle. The tendon crosses the calf relatively proximal, running medial from, and parallel with the Achilles tendon from the midportion of the calf, in the majority of cases ultimately inserting medially onto the calcaneus (Daseler 1943; Harvey et al. 1983). The plantaris muscle-tendon complex is a weak ankle and knee flexor and ankle invertor.
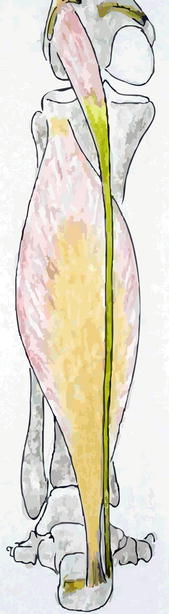
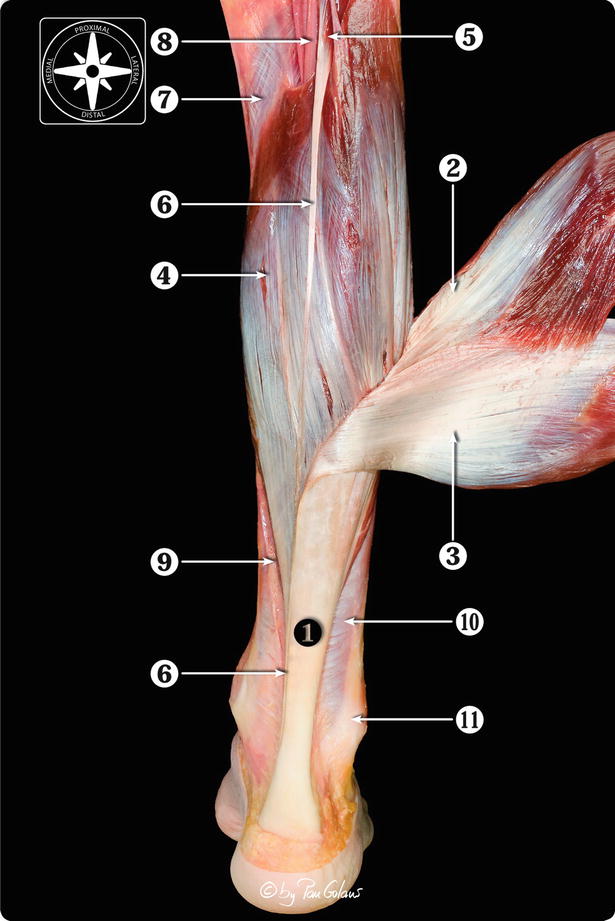

Fig. 22.2
Schematic drawing from posterior after removal of the gastrocnemius muscle. We see the gastrocnemius insertion on the medial and lateral femur condyle and the lateral insertion on the calcaneus. Because the gastrocnemius has been taken away, we have a direct view on the soleus which attaches to the posterolateral proximal tibia and to the medial calcaneus. Medial from it we see the plantaris tendon which inserts on the lateral femur condyle and runs anteromedial to insert on the calcaneus

Fig. 22.3
Posterior view of the anatomical dissection of the triceps surae muscle and its components. 1 Calcaneal or Achilles tendon. 2 Lateral head of the gastrocnemius muscle. 3 Medial head of the gastrocnemius muscle. 4 Soleus muscle. 5 Plantaris muscle belly. 6 Plantaris tendon. 7 Popliteus muscle. 8 Posterior neurovascular bundle (tibial nerve and posterior tibial artery and veins). 9 Medial septum of the leg. 10 Deep crural fascia. 11 Peroneal tendons (With kind permission of © Pau Golanó 2013)
It is believed that in a healthy situation the plantaris tendon can move freely in relation to the Achilles tendon. Adhesions between the plantaris and Achilles tendons can arise from an inflammatory response of the peritendineum, within which both Achilles and plantaris tendons run. The medial portion of the Achilles tendon consists solely of the soleus tendon, since at the level where the soleus contributes fibers to the Achilles tendon (3–11 cm), rotation of the tendon begins and becomes more marked in the distal 5–6 cm. Gastrocnemius fibers are therefore positioned lateral and the soleus fibers medial to the insertion (Fig. 22.4).
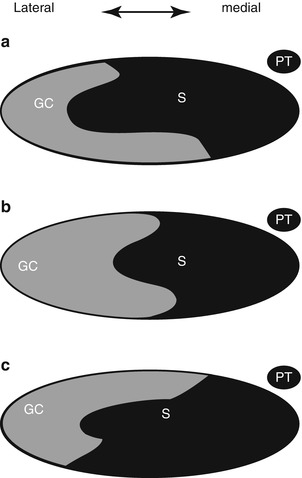

Fig. 22.4
Degree of rotation and position of the gastrocnemius-soleus complex at the midportion of the Achilles tendon. (GC gastrocnemius, S soleus.) The most common (52 %) position is depicted in (a). (b) Occurs in 35 %, and (c) in 13 %. In all the soleus fibers are positioned medially (Adapted from Cummins et al. (1946))
This anatomical observation means that the medial portion of the Achilles tendon, where pain is often most prominent, is monoarticular since it consists of fibers originating from the soleus muscle. Additionally, the plantaris muscle-tendon complex not only causes flexion but also inversion, whereas the triceps surae is a flexor only. Adhesions between both tendons will obstruct the opposite forces of the mono- and biarticular muscles that could well cause part of the pain and stiffness on walking (Van Sterkenburg et al. 2011a).
If conservative measures fail, surgery must be considered. The percentage of patients requiring surgery is 25 % (Kvist 1991; Maffulli 2005; Maffulli et al. 2008). The technique used for operative management of tendinopathy depends on the stage of the disease. Local degeneration and thickening are usually treated by excision and curettage. An insufficient Achilles tendon due to extensive degeneration can be reconstructed. Isolated paratendinopathy can be treated by excision of the diseased paratenon.
Open surgery has a guarded prognosis and is associated with a higher risk of complications when compared to endoscopic treatment (Abramowitz et al. 2003; Hamilton et al. 1996; Marotta and Micheli 1992). Open techniques are associated with an extensive rehabilitation period of 4–12 months. Recently minimally invasive techniques were developed.
In combined tendinopathy and paratendinopathy, the question is whether both pathologies contribute to the complaints. An anatomical cadaver study described degenerative changes of the Achilles tendon in as much as 34 % of subjects with no complaints (Kannus and Jozsa 1991). Khan and coworkers only found abnormal morphology in 65 % (37 of 57) of symptomatic tendons but also in 32 % (9 of 28) of asymptomatic Achilles tendons assessed by ultrasound (Khan et al. 2003). Therefore, it is questionable whether degeneration of the tendon itself is the main cause of the pain.
We focus on management of the paratendinopathy leaving the nodular thickened tendon proper untouched even when an intratendinous lesion is present. The current approach is release of adhesions of the peritendineum, denervation of the tendon, removal of pathological tissue of the peritendineum, and endoscopic release or resection of the plantaris tendon at the level of the complaints. The level of complaints invariably correlates with the localized nodule in the Achilles tendon.
22.3 Etiology
Achilles tendinopathy is common in sports-active individuals, especially in runners and badminton players. Overuse is thought to be the major cause of this problem, the prevalence of which is increasing because of the current keep-fit culture and the high demands which athletes impose on themselves. Since increasing scientific evidence has been published over the last three decades, advice for prevention of injuries can be specified. This includes adaptation of training methods and optimizing muscle strength, flexibility, and shoewear. Further research will have to determine the exact etiology of Achilles tendinopathy to be able to optimize treatment and prevention (Van Sterkenburg et al. 2010c, d).
22.4 Risk Factors for Achilles Tendinopathy
The exact causes of overuse injuries have yet to be determined, but it can be stated with certainty that it is multifactorial and diverse in the sports active (Marti et al. 1988; Rolf and Movin 1997; Van Mechelen 1995). In this part, we report the main etiological/risk factors.
22.4.1 Overuse
The most commonly reported cause of injury to runners is “too much, too soon” (Fahlstrom et al. 2002; Kvist 1991). Fahlström changed this statement into “too much all the time,” due to the fact that a high weekly training load was the only factor that correlated with pain in the Achilles tendon region in his study. James and coworker reported that 50 % of running-induced injuries in their study were caused by training errors. Errors can be excessive intensity and duration of running and are commonly associated with a failure to allow physiological adaptation (Rolf and Movin 1997).
However, it is unlikely that an elite runner would have progressed to a professional level with poor running technique.
Also, training mileage is a known risk factor for lower extremity injuries in exercising adults (Blair and Oberman 1987; Bovens et al. 1989; Caselli and Longobardi 1997; Hootman et al. 2002; Jacobs and Berson 1986; Koplan et al. 1982; Lysholm and Wiklander 1987; Macera et al. 1989; Marti et al. 1988; McQuade 1986; Walter et al. 1989). Hootman and coworkers found that running over 20 miles/week increased the risk of lower extremity injury by 71 % in men and 112 % in women (Walter et al. 1989). Loss of fitness because of winter-stop or preseason after which activities are not resumed at slower pace is also a predisposing factor for Achilles tendinopathy (Hawkins and Fuller 1999).
22.4.2 Previous Injury
Previous lower extremity injuries (mostly foot (22 %) and knee (24 %)) are known risk factors of subsequent injuries (2.0–7.6 times higher) (Macera et al. 1989). The less time between a previous injury and the current injury and the shorter the follow-up period between baseline and injury, the higher the risk of a new injury (Hootman et al. 2002; Macera et al. 1989; Van Gent et al. 2007).
22.4.3 Alignment Lower Extremity
Runners who have a variety of biomechanical variations in terms of alignment of the lower extremities are more vulnerable to injury. Genu varum, genu valgum, tibial torsion, pes planovalgus, and pes cavus are considered predisposing factors (Clement and Taunton 1981; Dubravcic-Simunjak et al. 2003). A gait cycle can be divided into the support phase and the forward recovery phase. Figure 22.5 describes the support phase by showing foot descent, heel strike, midstance, and toe-off.
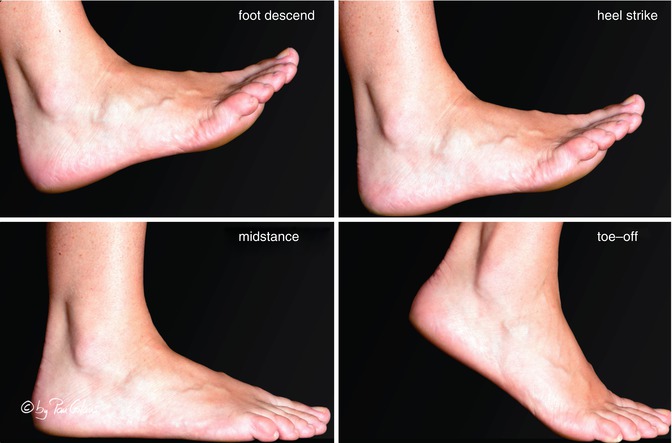

Fig. 22.5
Support phase in walking (With kind permission of © Pau Golanó 2013)
The foot prepares for descent by assuming a supine position. Following heel strike, the foot pronates which reaches a maximum in midstance. During this phase, the foot absorbs much of the shock energy of landing. Resupination starts in midstance. The foot becomes a rigid handle by locking the midtarsal joint facilitating the toe-off phase. Supination involves a complex movement of the ankle, subtalar, and midtarsal joints, which is composed of plantar flexion, inversion, and adduction. Pronation is composed of dorsiflexion, eversion, and abduction.
A pes planovalgus is often combined with a forefoot varus in the neutral position but when compensated during weight bearing will appear as a rearfoot valgus and a “low arch.” The cavus foot will show a forefoot valgus when unloaded, but when compensated during weight bearing, it will appear as a rearfoot varus and a “high arch.” A cavus foot will have a more lateral heel strike which results in a longer pronation distance in the midstance phase, in which the Achilles tendon has to make a an elongated medial swing, therefore absorbing excessive shock. A valgus foot causes eccentric pull on the Achilles tendon because of overpronation.
22.4.4 Terrain
Training on uneven grounds or an unstable terrain, for example, in adventure racing, has been described as a predisposing factor (Clement et al. 1979; Clement and Taunton 1981; Fordham et al. 2004).
In a systematic review, the incidence and determinants of lower extremity running injuries in long-distance runners were studied. There was limited evidence for an association between female runners running on concrete surfaces and lower extremity injuries (Macera et al. 1989; Van Gent et al. 2007). There was no significant association between male runners running on a specific surface and lower extremity injuries and between training on hilly terrain or running in the dark or in the morning and these injuries, implying that there is no association between these factors and lower extremity running injuries (Macera et al. 1989; Van Gent et al. 2007).
22.4.5 Temperature
One report among recruits mentioned a significant increase in the risk of sustaining “peritendinitis” when training outdoors in cold weather, with an incidence of 9.4 % in winter and 3.6 % in summer (Van Gent et al. 2007). We could not find any further scientific evidence on parallels with the etiology of Achilles tendinopathy.
22.5 Anatomy
The anatomy of the Achilles tendon is different from that of other tendons inserting into the foot. It lacks a true synovial sheath but rather has a paratenon. The paratenon functions as an elastic sleeve and permits free movement of the tendon within the surrounding tissues. The paratenon fills the space between the tendon and crural fascia (Figs. 22.6 and 22.7). Under the paratenon, the entire Achilles tendon is surrounded by a fine, smooth connective tissue sheath called the epitenon. On its outer surface, the epitenon is in contact with the paratenon. The inner surface of the epitenon is continuous with the endotenon, which binds the collagen fibers and fiber bundles together.
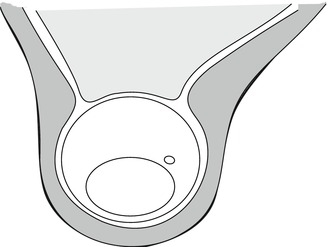
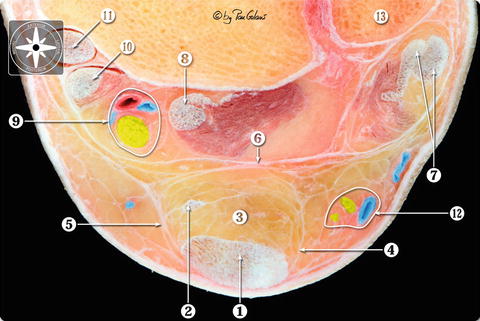

Fig. 22.6
Schematic cross section of the Achilles tendon compartment. We see the Achilles tendon, the plantaris tendon surrounded by the peritendineum, and the crural fascia. Between the crural fascia and the skin, a thin subcutaneous layer

Fig. 22.7
Transversal section of the calcaneal tendon compartment. We see the calcaneal or Achilles tendon and the plantaris tendon surrounded by the peritendineum and the crural fascia. Between the crural fascia and the skin, a thin subcutaneous layer. 1 Calcaneal tendon or Achilles tendon. 2 Plantaris tendon. 3 Kager’s fat pad. 4 Lateral crural septum and peritendineum. 5 Medial crural septum and peritendineum. 6 Deep crural fascia or fibulotalocalcaneal ligament (Rouvière and Canela ligament). 7 Peroneal tendons. 8 Flexor hallucis tendon. 9 Posterior neurovascular bundle (tibial nerve and posterior tibial artery and veins). 10 Flexor digitorum tendon. 11 Tibialis posterior tendon. 12 Sural nerve and small saphenous nerve. 13 Lateral malleolus (With kind permission of © Pau Golanó 2013)
The paratenon is richly vascularized and provides blood supply to the Achilles tendon itself. The neural supply to the Achilles tendon and the surrounding paratenon is provided by nerves from the attaching muscles and by small fasciculi from cutaneous nerves, in particular the sural nerve. The number of nerves and nerve endings is relatively low, and many nerve fibers terminate in the paratenon or on the tendon surface. These nerves follow the vascular channels within the long axis of the tendon, anastomose via obliquely and transversely oriented fibers, and finally terminate in sensory nerve endings. Achilles paratendinopathy involves inflammation of the peritendinous tissues. In patients with Achilles tendon overuse injury, the sensory nerve endings accompany the paratendinous neovascularization.
22.6 History and Physical Examination
Symptoms exist of pain and swelling around the Achilles tendon which often increase with activity in patients with isolated paratendinopathy. Patients with tendinopathy have tendon pain, localized swelling, and impaired performance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








