Fig. 15.1
Increased abdominal pressure affects multiple organ systems
Intra-abdominal hypertension also has a direct compressive effect on the renal system. This results in a relative obstruction to renal venous drainage plus an increase in renal vascular resistance. With a consequent reduction in blood flow, there is a decrease in urine output. There is a similar impact on the vascular system causing a reduction in hepatic and intestinal perfusion. Finally, there is an apparent association between ACS and intracranial hypertension, postulated to be due to a decrease in venous return.
Diagnosis
Clinical indices of end-organ derangement such as decreased urine output, increased pulmonary pressures, decreased preload, cardiac dysfunction, and elevated intracranial pressure are fundamental to the identification of ACS [26]. In any critically injured patient, however, there are many etiologies that could cause a low urine output and cardiopulmonary woes. Therefore, the diagnosis of IAH and ACS has to remain in one’s differential in the at-risk patient. Physical examination cannot definitively diagnose IAH or its severity [27]. Although the patient may have a markedly distended abdomen that is suggestive of the diagnosis, exam may reliable only about 40 % of the time. A diagnosis of IAH is obtained by measuring the patient’s bladder pressure. The bladder is capable of transmitting intra-abdominal pressure without imparting any additional pressure from its own musculature, hence acting as a passive reservoir. To measure a patient’s bladder pressure, 50 cc of saline is instilled into the bladder via the aspiration port of a 3-way Foley catheter with the drainage tube clamped; after waiting for 30–60 s to allow the detrusor musculature to relax, pressure measurement with a manometer at the pubic symphysis is performed [28]. Although this technique is a single measurement, continuous monitoring is also an option [29]. There are several conditions in which the bladder pressure may not be reflective of the intra-abdominal pressure. These include patients with external compression on the bladder such as pelvic packing, those with bladder rupture, marked adhesive disease, and patients with a neurogenic bladder.
A grading system based on bladder pressure measurements was developed to aid in the diagnosis and subsequent treatment of ACS (Table 15.1) [6]. Measurements are obtained in either cmH2O or mmHg depending upon the institution. Typically, intra-abdominal pressures over 20 cmH2O become worrisome. More recently, abdominal perfusion pressure, defined as the mean arterial pressure minus the intra-abdominal pressure, has been advocated to diagnose IAH and ACS [30]; to date this has not been widely adopted in practice. Recognizing that ACS is a late event in the evolution of IAH, monitoring at-risk patients is advocated; this allows one to intervene in patients with IAH in an attempt to prevent the sequelae of ACS. Maxwell and colleagues report that monitoring for IAH should occur after 10 units of packed red cells or 10L of crystalloid [11]. Others have also reported on the need for a heightened awareness of IAH with early monitoring of high-risk patients [9, 12, 31].
Table 15.1
Acute compartment syndrome grading system based on bladder pressure measurements
ACS grade | Bladder pressure | |
|---|---|---|
mmHg | cmH2O | |
I | 10–15 | 13–20 |
II | 16–25 | 21–35 |
III | 26–35 | 36–47 |
IV | >35 | >48 |
Intervention
There is not, unfortunately, a single pressure measurement that mandates intervention; rather, it is the combination of the bladder pressure measurement and end-organ sequelae (decreased urine output, increased pulmonary pressures, and decreased cardiac output) that is required for the diagnosis of ACS. Organ failure can occur over a wide range of recorded bladder pressures. If the patient has ACS, however, emergent decompression is indicated; mortality is directly affected by decompression [30].
Decompression is typically performed via a midline laparotomy incision performed in the operating room; this allows egress of peritoneal fluid or blood as well as evisceration of the edematous bowel (Fig. 15.2). In patients who are too unstable for transport to the operating room, operating room personnel and equipment can be transported to the ICU for a bedside procedure. Bedside laparotomy is easily accomplished, precludes transport in hemodynamically compromised patients, and requires minimal equipment (scalpel, suction, cautery, and abdominal temporary closure dressings). Patients with significant intra-abdominal fluid as the primary component of their ACS may be candidates for decompression via a percutaneous drain [31–33]. Differentiation of those amenable to such drainage is determined by bedside ultrasound, hence obviating a trip to the operating room. Removing a significant amount of ascites can lower the intra-abdominal pressures enough to obviate laparotomy.
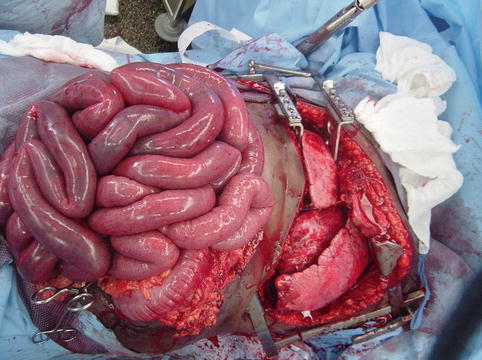

Fig. 15.2
Midline decompressive laparotomy performed for secondary abdominal compartment syndrome following massive resuscitation for a gunshot wound to the heart; peritoneal fluid or blood is evacuated, and the edematous bowel protrudes from the abdominal cavity
Following operative decompression, temporary coverage of the abdominal viscera is necessary. One option of temporary closure is “towel clipping” the abdomen; penetrating towel clips are placed through the skin 2–3 cm apart to approximate the abdominal wall over the length of the laparotomy incision (Fig. 15.3). Although this rapidly closes the abdomen, the closure does not allow egress of the edematous bowel which may promote recurrent ACS. Another option for temporary closure is the Bogota bag closure (Fig. 15.4). This temporary silo is constructed of a sterile 3L GU irrigation bag or X-ray cassette cover that is sutured to the skin; this contains the edematous bowel while providing excellent decompression. A third option for temporary coverage is a vacuum-assisted closure device (Fig. 15.5); the caveat, however, is to ensure that recurrent ACS does not occur due to the application of suction-related pressure on the viscera. Our preferred option for temporary closure is 1010 Steri-Drape and Ioban closure (Fig. 15.6). In this technique, the bowel is covered with a fenestrated subfascial 1010 Steri-Drape (3M Health Care, St. Paul, MN); small holes are cut in the plastic drape with a scalpel to allow fluid to pass through the drape while not allowing the Ioban to stick to the underlying bowel. The drape is placed over the bowel and tucked under the fascia. Two Jackson-Pratt drains, with the tubing running cephalad, are placed along the fascial edges to control reperfusion-related ascitic fluid. Everything is then covered using a large Ioban (3M Health Care, St. Paul, MN). In any temporary closure of the abdomen, one has to ensure that the patient does not develop recurrent ACS; one should not assume that because the patient has an open abdomen that they cannot develop IAH or ACS [34]. Therefore, leaving “expansion space” for the bowel in your temporary covering is critical. Additionally, monitoring bladder pressures in patients who are unstable or have low urine output is an important adjunct.
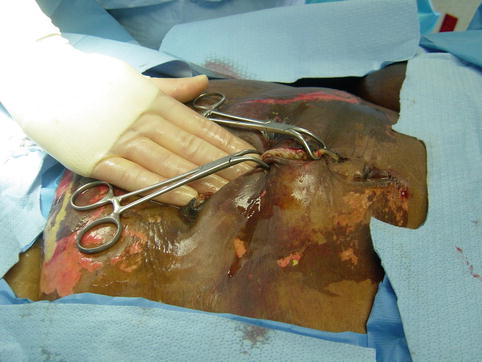
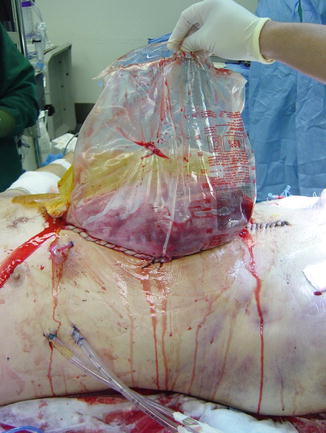
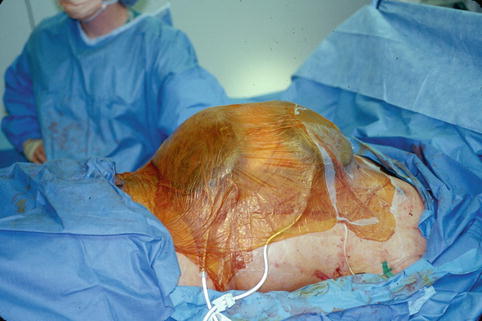
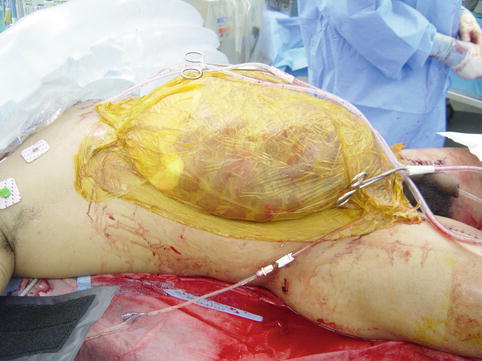

Fig. 15.3
Towel clip closure of the abdominal skin

Fig. 15.4
A sterile 3L GU irrigation bag sutured to the skin, also termed the Bogota bag closure of the abdomen, permits egress of the edematous viscera

Fig. 15.5
Vacuum-assisted closure with applied suction may be used to cover the abdominal contents

Fig. 15.6
1010 Steri-Drape and Ioban temporary closure of the abdomen
ICU Management of the Open Abdomen
Management of the patient with an open abdomen is not markedly different from the care of any critically ill patient. The guidelines in such cases include appropriate resuscitation, rewarming, correction of acidosis and coagulopathy, lung protective ventilation strategies (once resuscitated), prevention of ventilator-associated pneumonia, treatment of adrenal suppression, and management of hyperglycemia. Issues specific to the open abdomen patient include balanced fluid administration, nutrition support, and management of enteric injuries.
During the acute resuscitation in the first 12–24 h following injury, initial volume loading attains adequate preload followed by judicious use of inotropic agents or vasopressors [35]. Optimizing fluid administration is a challenging aspect of early patient care, balancing cardiac performance versus generating marked visceral and retroperitoneal edema. Supranormal trauma resuscitation has been shown to require more crystalloid administration and to cause more cases of ACS [36]; therefore, goal-directed therapy should aim for an oxygen delivery index of >500 mL/min/m2 [37]. Although early colloid administration may be appealing in these patients, evidence to date does not support this concept [38].
Despite studies illustrating the importance of enteral nutrition (EN) in the trauma population [39–44], there remains hesitancy about enteral feeding in post-injury patients with an open abdomen. This may relate to issues of enteral access, concerns about bowel edema, or questions of intestinal motility and enterocyte functionality. Three studies addressing EN in the open abdomen patient have conflicting findings regarding impact of EN on abdominal closure rates and septic complications [45–47]. The most recent evaluation of feeding patients with an open abdomen [48] demonstrated EN in patients with bowel injuries did not appear to alter fascial closure rates, complications, or mortality; hence, EN appears to be neither advantageous nor detrimental in these patients. For patients without a bowel injury, EN in the open abdomen was associated with a marked increase in successful fascial closure, a decrease in complications, and a decrease in mortality. That study concluded that EN in the post-injury open abdomen was feasible. Therefore, once resuscitation is complete, initiation of EN should be considered in all injured patients [49].
Abdominal Closure
Coverage of the enteric contents is the most critical step in the management of the open abdomen. Leaving the bowel exposed to the atmosphere can result in enteroatmospheric fistulas which are notoriously difficult to manage. The ideal coverage for the bowel is native fascia, so primary closure is the goal, either with early fascial closure or sequential fascial closure techniques. Other options for bowel coverage include prosthetic fascial closure with either mesh or biologics, or bowel coverage with skin grafts and planned ventral hernia.
Our preferred approach in Denver for those patients that are not closed at second laparotomy is the sequential fascial closure technique [50, 51], a modification of Miller et al.’s described VAC technique [52]. In our described technique, closure is sequentially performed with the combination of a Wound VacTM as well as constant fascial tension with sutures (Fig. 15.7). Patients are returned to the operating room for sequential fascial closure and replacement of the sponge sandwich every 2 days until closure is accomplished. Gastrostomy and needle catheter jejunostomy tubes may be placed prior to complete closure; however, manipulation or marked movement of enteral access sites could cause injury with fistula formation. For this reason, operative gastrostomy and jejunostomy tubes should not be placed until closure of the fascia is well under way. There may be hesitancy to place an operative jejunostomy through the edematous bowel wall; however, this can be safely performed [49]. Alternatively, nasojejunal access is also a viable option for early enteral nutrition.
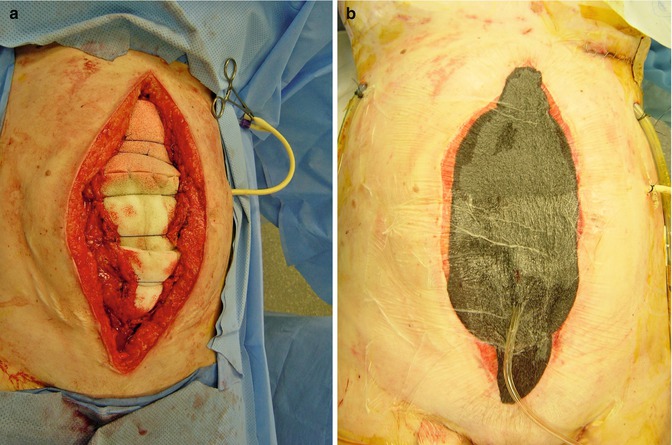

Fig. 15.7




Sequential fascial closure technique uses a sponge sandwich of white sponges over the bowel with #1 PDS sutures over the white sponges to prevent fascial retraction (a); black sponges, occlusive dressing and standard suction is applied on top of the white sponges (b)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







