Update on Vasculitis
Emory University School of Medicine, Atlanta, GA, USA
Introduction
The vasculitides are a heterogeneous set of diseases identified by the presence of blood vessel wall inflammation, with associated tissue and organ damage specific to each disease. Several classification schemas exist; in general the vasculitides are classified based on the size and type of blood vessels affected, as well as the presence or absence of immune deposits and ANCA (Figure 13.1). While, classically, many vasculitides have been known by their eponyms, recent consensus groups recommend a shift towards disease-specific nomenclature. This includes the identification of Churg–Strauss vasculitis as eosinophilc granulomatous polyangiitis (EGPA) and Wegener’s granulomatosis as granulomatous polyangiitis (GPA). This chapter will focus on recent updates to the ANCA-associated vasculitides (AAV).
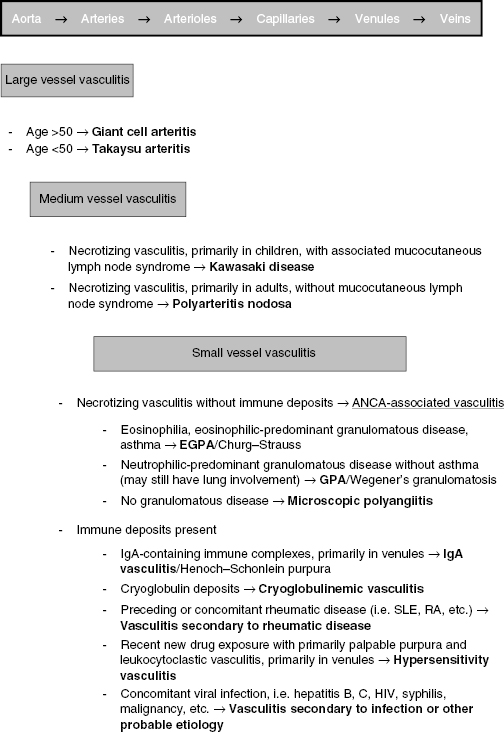
ANCA-Associated Vasculitides
The ANCA-associated vasculitides include the following:
- Granulomatosis with polyangiitis (GPA, previously Wegener’s granulomatosis)
- Microscopic polyangiitis (MPA)
- Eosinophilic granulomatosis with polyangiitis (EGPA, previously Churg–Strauss syndrome)
What are ANCAs?
- ANCAs are serologic markers for AAV, and are autoantibodies directed against granular and lysosomal components of neutrophils
- Fluorescent staining patterns are determined by indirect immunofluorescence and distinguish cytoplasmic ANCA (C-ANCA) staining patterns from perinuclear (P-ANCA) staining patterns
- Direct testing of target antigens such as proteinase-3 (PR3) and myeloperoxidase (MPO) is often performed after C-ANCA or P-ANCA is detected by indirect immunofluorescence
- The target antigen of C-ANCA is PR3
- The target antigen of P-ANCA is MPO
- PR3-ANCA are the predominant ANCA type in GPA, while MPO-ANCA are the predominant ANCA type in MPA and EGPA, though overlapping or atypical ANCA expression can be seen
- The target antigen of C-ANCA is PR3
- ANCAs are hypothesized to have a pathogenic role in the tissue inflammation and vascular injury seen in AAV
- In vitro studies have shown that proinflammatory cytokines stimulate neutrophils to express ANCA target antigens on their cell surfaces; these neutrophils, when exposed to ANCA, degranulate, releasing enzymes that may induce vasculitic lesions
- Animal studies have shown that infusion of anti-MPO IgG induces small vessel vasculitis including glomerulonephritis and pulmonary capillaritis
- Limited case studies have shown that transplacental transfer of anti-MPO ANCA can result in neonatal pulmonary-renal disease
- In vitro studies have shown that proinflammatory cytokines stimulate neutrophils to express ANCA target antigens on their cell surfaces; these neutrophils, when exposed to ANCA, degranulate, releasing enzymes that may induce vasculitic lesions
- New data suggest a role for antibodies to LAMP-2, a lysosomal membrane protein, in the pathogenesis of AAV
- LAMP-2 is expressed on the intracellular vesicles of neutrophils, especially those vesicles containing MPO and PR3
- Scientists hypothesize that exposure to an infectious agent may induce autoantibodies to LAMP-2 via molecular mimicry, thereby inducing neutrophil activation and expression of disease
- Although reports vary, the presence of autoantibodies to LAMP-2 has been reported in up to 90% of patients with AAV, with a rapid decline in antibody levels between disease flares
- Additional studies are necessary to fully characterize the role of anti-LAMP-2 antibodies in AAV
- Although reports vary, the presence of autoantibodies to LAMP-2 has been reported in up to 90% of patients with AAV, with a rapid decline in antibody levels between disease flares
- LAMP-2 is expressed on the intracellular vesicles of neutrophils, especially those vesicles containing MPO and PR3
Treatment of AAV
Conventional Treatment of AAV
- For decades, corticosteroids with traditional immunosuppressants, such as cyclophosphamide, have been the foundation of induction therapy in AAV, followed by maintenance therapy with azathioprine or methotrexate
- Cyclophosphamide may be given PO or IV
- Rates of remission are comparable between the two dosing routes
- Monthly IV cyclophosphamide is associated with a lower total cyclophosphamide dose and lower rates of leukopenia, but higher relapse rates
- Rates of remission are comparable between the two dosing routes
- Both methotrexate and azathioprine are equivalent in maintaining remission after induction therapy
- Mycophenolate mofetil has been shown in one study to be inferior to azathioprine in preventing relapse and therefore is not a first-line agent
- Cyclophosphamide may be given PO or IV
- In patients with severe, active renal disease, limited data supports the addition of plasma exchange to traditional induction regimens of cyclophosphamide and corticosteroids; expert opinion also suggests a role for plasma exchange in the setting of pulmonary hemorrhage, though randomized studies have not been performed to establish superiority over standard care
- These regimens have improved survival, but are also associated with serious treatment-related adverse events; frequent relapses also add to substantial morbidity
- Adverse events include:
- Bone marrow suppression
- Infertility
- Malignancy, especially bladder cancer and lymphoma
- Serious infection
- Cystitis
- Bone marrow suppression
- Adverse events include:
Rituximab in the Treatment of AAV
- Two prospective, randomized controlled trials have examined rituximab for the treatment of AAV
- The RAVE trial established the non-inferiority of rituximab 375 mg/m2 IV weekly for 4 weeks compared to oral cyclophosphamide in the induction therapy of AAV
- Both groups received a similar regimen of pulse corticosteroids followed by a tapering corticosteroid regimen
- Rituximab was found not to be inferior to cyclophosphamide (p < 0.001) at the primary endpoint on a standardized vasculitis activity scale (the Birmingham Vasculitis Activity Score)
- There were no significant differences in adverse events between the two groups
- Patients receiving treatment for relapsing disease had superior outcomes in the rituximab arm when compared to the cyclophosphamide arm
- The RITUXVAS trial, run concomitantly to the RAVE trial, also established the non-inferiority of rituximab weekly for 4 weeks with two IV cyclophosphamide doses compared to standard IV cyclophosphamide for 3–6 months followed by azathioprine in patients with AAV and severe renal disease
- The rituximab group was found to be non-inferior to the cyclphosphamide group at the primary endpoint of remission at 12 months (76% of patients in the rituximab arm vs 82% in the cyclophosphamide arm, p = ns)
- There were no significant differences in adverse events between the two groups
- The bottom line
- Rituximab is a promising alternative to cyclophosphamide in induction therapy for moderately active AAV, and in 2011 became the first FDA-approved treatment for AAV
- Rituximab may spare patients the toxicities customarily associated with cyclophosphamide, such as infertility, hemorrhagic cystitis, bone marrow suppression, and malignancy
- Rituximab does appear to be superior to cyclophosphamide in patients with relapsed disease
- The role of concomitant cyclophosphamide with rituximab (as in the RITUXVAS trial) needs to be further investigated
- Maintenance therapy after rituximab has not yet been well established; in the RITUXVAS and RAVE studies, no maintenance therapy was given
- While rituximab represents a paradigm-shifting development in the treatment of AAV, additional long-term studies are needed to address remaining questions, including:
- Rates of relapse after rituximab
- Long-term adverse effects
- Approach to re-treatment when a relapse occurs
- Role of rituximab or other agents in maintenance therapy
- Role of rituximab in severely ill patients with renal or respiratory failure
- Rates of relapse after rituximab
- The RAVE trial established the non-inferiority of rituximab 375 mg/m2 IV weekly for 4 weeks compared to oral cyclophosphamide in the induction therapy of AAV







