Fig. 10.1
Expose the L4, L5 and L6 nerve root, each was indicated by a line
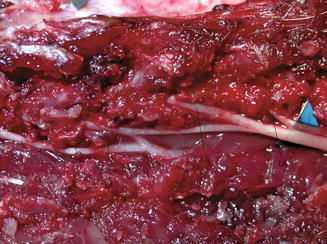
Fig. 10.2
Completely explosion, each nerve root was indicated by a line
10.2.2.2 Electrophysiological Studies
Following induction of anesthesia, the rat was placed in the prone position. The stimulation electrodes were hooked to the sciatic nerve and the recording electrodes were located in the triceps surae, anterior tibialis and biceps femoris, respectively, to record EMGs. One recording electrode was buried in the muscle belly, another in the muscle tendon, and the remaining electrode was fixed to the gluteus maximus as an extra ground electrode. The compound muscle action potentials of the triceps surae, anterior tibialis and biceps femoris were recorded with a stimulating intensity of 1 mA, a pulse width of 0.2 ms, a frequency of 1 Hz, and duration of 10 ms. Before one of the right L4, L5 and L6 nerve roots was severed in each experimental group, the sciatic nerve was stimulated and the compound muscle action potentials of the triceps surae, anterior tibialis and biceps femoris were recorded on the computer. After the nerve root was severed, the same operation was performed and the compound muscle action potentials were again recorded. Then, at 4, 8, and 12 weeks post-operation, the sciatic nerve was stimulated and the compound muscle action potentials were recorded by the same process.
All signals were imported to a computer and analyzed using MFLab software version 3.01 (Department of Physiology and Pathophysiology, School of Medicine, Fudan University, Shanghai, China).
10.2.2.3 Sciatic Functional Index (SFI)
Pre- and post-operation (4-, 8-, and 12-weeks), walking tracks were obtained before induction of anesthesia. Animals were tested in a confined walkway 42-cm in length and 8.2-cm in width, with a dark shelter at the end. A sheet of white paper was placed on the floor of the walking corridor. After the hind paws of the rats were pressed down onto a paint-soaked sponge, they were allowed to walk down the corridor, leaving hind footprints on the paper. In each walking track, three footprints were analyzed by a single observer, and the average of the measurements was used in SFI calculations. Several measurements were taken from the footprints: (I) distance between heel and third toe, print length (PL); (II) distance between the first and the fifth toe, the toe spread (TS); and (III) distance between the second and the fourth toe, the intermediary toe spread (ITS). All three measurements were taken from the right (experimental) sides. The SFI was calculated according to the following formula, as described by Bain et al. [15]:
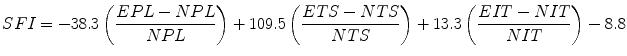
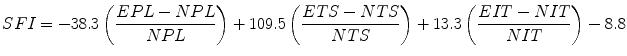
The SFI oscillates around 0 for normal nerve function, and a figure of around −100 indicates total dysfunction.
10.2.2.4 Wet Weight of Muscles
Following the electrophysiological examination 12 weeks after severing of the nerve, the right triceps surae, anterior tibialis and biceps femoris were dissected out from bilateral hind limbs and weighed while wet. After weighing, the muscles were fixed with 4 % paraformaldehyde in phosphate-buffered saline (PBS, pH 7.4) at 4 °C.
10.2.2.5 Electron Microscopic Observation of the Ultrastructure of the Target Muscle and Motor End-Plate (MEP)
The triceps surae, anterior tibialis and biceps femoris were double-fixed in 4 % glutaraldehyde and osmic acid, dehydrated with pyroracemic acid, and embedded in EPON812. They were then sliced using a LKBNOVA ultramicrotome (LKB Inc., Bromma, Sweden), double-dyed with lead and uranium and observed using a Philips CM120 transmission electron microscope (Philips Inc., Amsterdam, The Netherlands) for ultrastructure of the triceps surae, anterior tibialis and biceps femoris and MEP.
10.2.2.6 Histological Examination
The triceps surae, anterior tibialis and biceps femoris were fixed in 10 % formalin for 40 h, dehydrated and paraffin-embedded. They were then sliced along the middle of the muscle into 5 mm sections, hematoxylin and eosin stained, and analyzed using the FW4000 digital imaging workstation (Leica Inc., Solms, Germany).
10.2.2.7 Fibrotic Components in the Target Muscle
To observe the fibrotic level, the triceps surae, anterior tibialis and biceps femoris were dyed with Masson’s trichrome. The sections were analyzed using the FW4000 digital imaging workstation (Leica Inc., Solms, Germany).
10.2.2.8 Statistics
Results were expressed as mean ± standard deviation, and SPSS 11.0 software (SPSS Inc., Chicago, IL) was used. One-way analysis of variance was used to determine significant differences between the groups, with the Student–Newman–Keuls test for multiple comparisons. Differences were considered significant at P < 0.05. We certify that all applicable institutional and governmental regulations concerning the ethical use of animals were followed during the course of this research.
10.2.3 Results
The animals in all groups survived for the duration of the experiments, without wound infection or self-eating of limbs.
10.2.3.1 Compound Muscle Action Potentials (CMPs)
Before and after severing the nerve, the mean latencies of CMP and maximum CMP amplitudes in groups A–D for the triceps surae, anterior tibialis and biceps femoris on the right side of rats (n = 10) were, assessed respectively. There were no significant differences in either the mean latency or mean maximum CMP amplitude among the four groups. At the 4th week after surgery, there were significant differences in the mean latency among the four groups. Then at the 8th and 12 week after surgery, the differences in either the mean latency or mean maximum CMP amplitude among groups A-D for the triceps surae, anterior tibialis and biceps femoris on the left side of the rats (n = 10) became insignificant (Tables10.1 and 10.2).
Table 10.1
Compound muscle action potentials (CMPs) (mv)
The triceps surae | Anterior tibialis | Biceps femoris | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Pre- | Post- | 4 Weeks | 8 Weeks | 12 Weeks | Pre- | Post- | 4 Weeks | 8 Weeks | 12 Weeks | Pre- | Post- | 4 Weeks | 8 Weeks | 12 Weeks | |
Control | 39.89 ± 9.13 | 39.02 ± 9.08 | 38.69 ± 10.52 | 39.17 ± 8.63 | 39.54 ± 11.69 | 29.71 ± 9.09 | 28.34 ± 11.48 | 28.91 ± 8.27 | 28.75 ± 10.59 | 28.59 ± 10.32 | 20.05 ± 4.34 | 18.01 ± 7.18 | 18.3 ± 5.67 | 18.59 ± 6.91 | 18.91 ± 4.37 |
L4 | 40.23 ± 10.34 | 39.56 ± 10.51 | 38.36 ± 9.42 | 38.91 ± 10.28 | 39.20 ± 11.15 | 30.12 ± 8.19 | 27.89 ± 8.34 | 27.13 ± 9.57 | 27.8 ± 9.24 | 27.95 ± 9.37 | 20.91 ± 3.82 | 18.51 ± 5.39 | 17.85 ± 3.28 | 18.37 ± 4.81 | 18.59 ± 3.85 |
L5 | 39.04 ± 10.29 | 39.19 ± 11.73 | 37.91 ± 10.75 | 38.27 ± 11.49 | 38.77 ± 7.83 | 29.52 ± 7.58 | 28.19 ± 9.88 | 26.89 ± 10.69 | 27.58 ± 9.37 | 27.87 ± 10.19 | 19.83 ± 5.19 | 17.94 ± 4.87 | 17.37 ± 5.14 | 17.96 ± 5.38 | 18.46 ± 4.76 |
L6 | 39.35 ± 9.68 | 39.21 ± 11.94 | 39.13 ± 11.43 | 39.77 ± 9.41 | 39.68 ± 9.37 | 30.34 ± 10.13 | 28.46 ± 7.22 | 28.04 ± 10.81 | 28.57 ± 8.61 | 28.96 ± 9.17 | 20.47 ± 4.98 | 18.35 ± 7.33 | 18.19 ± 4.79 | 18.43 ± 4.69 | 18.89 ± 5.19 |
Table 10.2
The mean latency (ms)
The triceps surae | Anterior tibialis | Biceps femoris | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Pre- | Post- | 4 Weeks | 8 Weeks | 12 Weeks | Pre- | Post- | 4 Weeks | 8 Weeks | 12 Weeks | Pre- | Post- | 4 Weeks | 8 Weeks | 12 Weeks | |
control | 1.42 ± 0.19 | 1.56 ± 0.16 | 1.81 ± 0.21 | 1.82 ± 0.17 | 1.79 ± 0.23 | 1.51 ± .24 | 1.53 ± 0.21 | 1.78 ± 0.24 | 1.77 ± 0.16 | 1.79 ± 0.13 | 1.54 ± 0.23 | 1.53 ± 0.27 | 1.69 ± 0.28 | 1.75 ± 0.24 | 1.83 ± 0.27 |
L4 | 1.51 ± 0.21 | 1.61 ± 0.23 | 2.14 ± 0.19 | 1.84 ± 0.27 | 1.76 ± 0.18 | 1.47 ± 0.19 | 1.49 ± 0.17 | 2.05 ± 0.19 | 1.84 ± 0.24 | 1.81 ± 0.27 | 1.49 ± 0.18 | 1.51 ± 0.15 | 2.01 ± 0.12 | 1.83 ± 0.19 | 1.81 ± 0.17 |
L5 | 1.49 ± 0.17 | 1.53 ± 0.17 | 2.26 ± 0.32 | 1.79 ± 0.13 | 1.74 ± 0.21 | 1.53 ± 0.23 | 1.46 ± 0.28 | 2.13 ± 0.26 | 1.86 ± 0.17 | 1.83 ± 0.16 | 1.57 ± 0.26 | 1.56 ± 0.17 | 2.17 ± 1.17 | 1.87 ± 0.23 | 1.83 ± 0.24 |
L6 | 1.56 ± 0.14 | 1.59 ± 0.19 | 1.76 ± 0.19 | 1.70 ± 0.24 | 1.78 ± 0.19 | 1.49 ± 0.18 | 1.54 ± 0.15 | 1.75 ± 0.29 | 1.74 ± 0.27 | 1.72 ± 0.28 | 1.49 ± 0.24 | 1.54 ± 0.26 | 1.77 ± 0.21 | 1.82 ± 0.17 | 1.76 ± 0.17 |
10.2.3.2 Sciatic Functional Index (SFI)
Reproducible walking tracks could be measured from all rats. Before severing the nerve, the mean SFI was assessed in groups A, B, C and D, with no significant differences observed (P > 0.05). At the 4th, 8th, and 12th week after surgery, the mean SFI was again assessed, with no significant differences found among the four groups at any of the time points (P > 0.05) (Table 10.3).
Table 10.3
Sciatic functional index (SFI)
Pre-operation | 4 Weeks | 8 Weeks | 12 Weeks
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|





