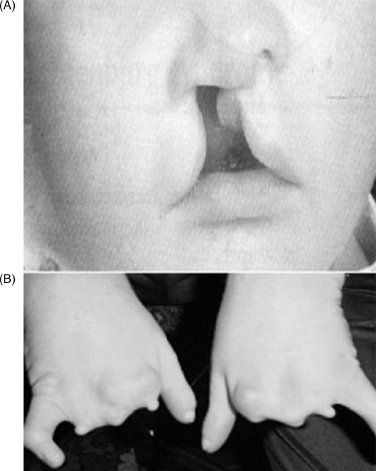
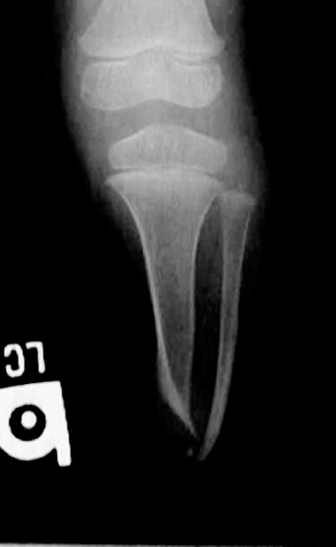
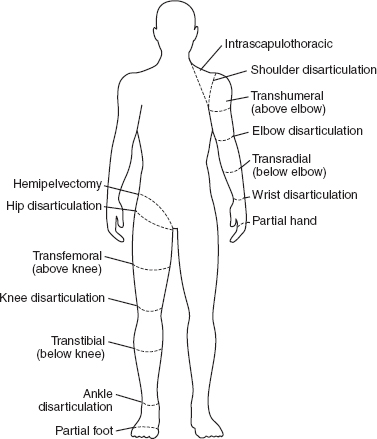
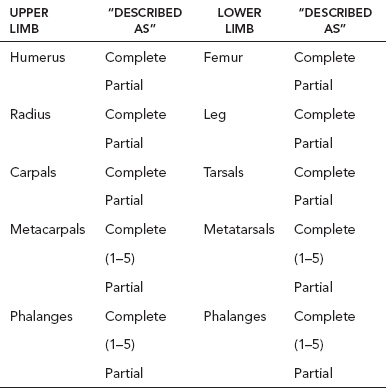
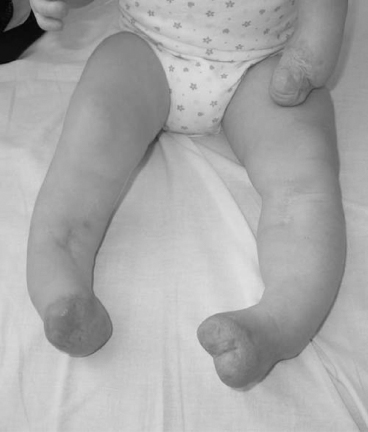
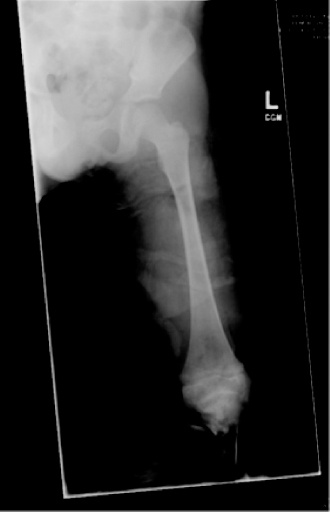
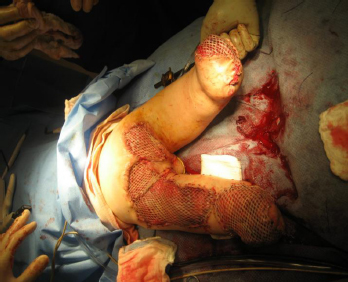
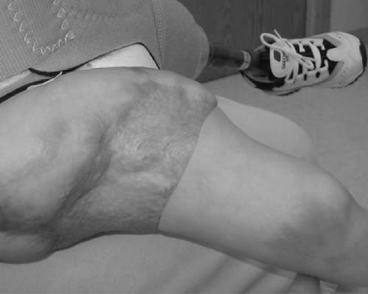
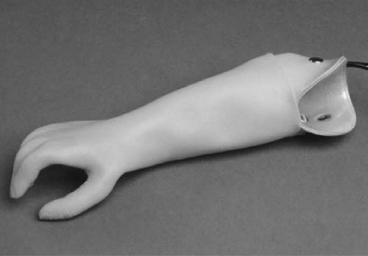
13 PEDIATRIC LIMB DEFICIENCIES Ann Modrcin, Matthew McLaughlin, and Matthew Luetke INTRODUCTION One of the more rewarding experiences in medicine comes from engaging children with limb deficiency in a clinical context. Patients and families experience varied outcomes and challenges, which allow providers the unique opportunity to positively intervene at various points in the patients’ lives with technology, innovation, and reassurance. Despite the challenges resulting from limb deficiency, the majority of these patients are socially well-adjusted, independent-minded, and able to interact well with providers, peers, and society. Treatment strategies in the pediatric limb deficiency population differ significantly from those of adults with amputations. In pediatrics, health care providers must consider the lifelong implications of choices made during childhood and examine the expected longitudinal timeline to achieve the best possible outcomes. Surgical considerations vary even among the pediatric age groups and may require expected revisions or in-depth, multidisciplinary discussion of surgical options. Rapid growth results in more frequent prosthetic adjustment and replacement to keep up with growth and developmental changes of a child. Prosthetic intervention should always augment rather than hinder a child’s function, whether for daily activities or for specific tasks, and sometimes the best device is none at all. CONGENITAL DEFICIENCIES INCIDENCE Epidemiologically, limb deficiencies occur in 5 to 9.7 births for every 10,000 live births, with a ratio of 3:1 upper to lower extremity anomalies (1). The United States does not have a formal complete registry of birth defects, so the precise number is unknown. The National Birth Defects Prevention Study has reported that 6% of all types of birth defects are limb deficiencies and the majority of limb deficiencies involve the upper extremity (2,3). Early identification of limb anomalies occurs with routine ultrasound. A detailed level 3 ultrasound, as well as echo three-dimensional (3D), amniocentesis, and cordocentesis to anticipate syndromes, is recommended if limb deficiencies are detected (4). Parents may experience a variety of emotions after learning of the diagnosis of limb deficiency. Prenatal counseling provides resources and psychological support for parents, and educates families about the process of expected functional level and potential prosthetic utilization. ETIOLOGY Although the presence of a limb deficiency evokes salient questions about etiology, frequently there is no identifiable cause of the limb deficiency (5). Embryologically, the first trimester is the most crucial trimester for the genesis of limb production. Congenital limb deficiency occurs as a result of failure of formation of part or all of the limb bud. The mesodermal formation of the limb occurs at 26 days’ gestation and continues with differentiation until 8 weeks’ gestation. The various limb segments develop in a proximal-to-distal order so that the arm and forearm appear before the hand, and the thigh and leg before the foot (6). Limb development is a complex process that involves orchestration of a number of genes, some of which are well known and studied. Known abnormalities account for various identified syndromes (7). A relatively small set of genes and gene families appear to control the early stages of limb development. More than 80% of heritable limb deficiencies are associated with anomalies outside the musculoskeletal system (7). When other anomalies are suspected, it is crucial to take an expanded history regarding feeding difficulties, respiratory distress, bowel dysfunction, and cardiac abnormalities (8). Compared to lower extremity limb deficiencies, upper limb deficiencies are more commonly associated with other anomalies, particularly craniofacial, cardiac, and hematologic disorders. This is due to the chronology of development in the first trimester (9). Bilateral deficiencies are more common with craniofacial abnormalities, whereas left–right asymmetry of organogenesis is more commonly associated with unilateral and left axial deficiency (10,11). Vascular pathology is not inherited, so the risk of recurrence is small (5,7). Despite this low risk of recurrence, the frequency of vascular issues contributing to limb deficiency is 34% (5). Conditions with implied vascular disruption include Adams–Oliver syndrome, gastroschisis, Klippel–Feil syndrome, Moebius syndrome, Poland syndrome/sequence, and terminal transverse limb deficiency (12–14). Two key features of Moebius are demonstrated in Figure 13.1: craniofacial anomalies and upper-limb deformity. FIGURE 13.1 Two key components of clinical presentation in Moebius syndrome: craniofacial (A) and upper limb (B) deformities. Other factors increase the risk for limb deficiency, including maternal diabetes, and gestational diabetes (15,16). Although alcohol, heroin, and cocaine have not been found to be related to limb deficiency, all maternal ingestions and first-trimester abnormalities should be documented during the initial visit (17,18). Smoking increases the risk of digit anomalies (19). Thalidomide historically presents a clear association with limb reductions (20). Case reports implicate valproic acid and calcium channel blockers (21,22). Maternal occupation may play a role, with exposure to chemicals, as in the agricultural setting (23). Uterine abnormalities have been reported in several cases of limb deficiencies, theoretically due to compression of the fetus (6). In addition, disturbances to the uterine environment, such as chorionic villus sampling, are implicated in deficiencies (24). Amniotic band syndrome is associated with fibrous bands that may constrict the limbs (25,26). Radiological findings of amniotic band are illustrated in Figure 13.2. Prenatal vitamins reduce the risk of limb deficiencies (27). Postnatal problems, such as gangrene from vascular emboli and neonatal injury from vascular compromise secondary to umbilical catheters, may necessitate immediate amputation (28–30). Although the perinatal causes for amputation may be different from congenital disorders, the clinical issues for the child and the rehabilitation team are more similar to congenital disorders than acquired disorders of later childhood. FIGURE 13.2 X-ray of hands affected by amniotic band syndrome. CLASSIFICATION The International Society for Prosthetics and Orthotics (ISPO) has adopted a definitive system for congenital deficiencies. This system describes limb deficiency on anatomic and radiological basis only (31). The utilization of this system aims to decrease ambiguity by more precisely classifying the type and location of the limb deficiency. First, the limb is classified as having either a transverse or longitudinal deficiency. No longer is it necessary to learn ancient language roots to describe limb deficiency (32–34). However, like an old language and culture, the terminology once used in clinics is difficult to change. Clinical teams often use a fusion of terms. Many clinics still describe deficiencies by the Frantz classification system (Table 13.1). In this system, deficiencies are either terminal, representing the complete loss of the distal extremity, or intercalary, denoting the absence of intermediate parts with preserved proximal and distal parts of the limb. Those deficits are then divided into horizontal and longitudinal deficits. TABLE 13.1 CLASSIFICATION OF LIMB DEFICIENCY ISPO/ISO FRANTZ–O’RAHILLY CLASSIC Longitudinal radius deficiency Intercalary radial deficiency Radial hemimelia/club hand Terminal transverse humerus deficiency Terminal horizontal humerus deficiency Above-elbow amputation ACQUIRED AMPUTATIONS TERMINOLOGY The terminology utilized for acquired amputations follows the convention for adult limb loss. Upper extremity amputations include intrascapulothoracic, shoulder disarticulation, transhumeral (above-elbow amputation), elbow disarticulation, transradial (below-elbow amputation), wrist disarticulation, and partial hand amputations. The types of lower extremity amputations are translumbar (hemicorpectomy), transpelvic (hemipelvectomy), hip disarticulation, transfemoral (above-knee amputation), knee disarticulation (through-knee), transtibial (below-knee amputation), ankle disarticulations (ie, Syme, Boyd, and Pirigoff), and partial foot (ie, Chopart and Lisfranc) (35). Figure 13.3 illustrates present classifications of acquired amputations. FIGURE 13.3 Classifications of acquired amputations. TRAUMATIC INJURIES In the pediatric age group, the most common causes of acquired amputations are trauma and disease (36). Trauma causes limb loss twice as often as disease (37). The most common traumatic injuries result from automobile and motorcycle collisions and train accidents. Causes for traumatic injuries vary with region. In rural areas, farm accidents, lawn mower accidents, and high-tension wire injuries occur more frequently (38–41). For the older child, vehicular accidents, burns, gunshot wounds, and power tools are the most frequent causes of limb loss. Boating accidents can produce amputations by propeller injury. Sadly, in the 1- to 4-year-old age range, power tools such as lawn mowers and household accidents are frequent mechanisms of amputation (42,43). Fortunately, in those traumatic events where digit or even hand replantation is possible, pediatric patients experienced improved functional and cosmetic outcomes with less frequent complications than their adult counterparts. However, patients identified as African American or Hispanic and those without insurance were less frequently noted in another study to have attempted replantation (44,45). For those injuries that require inpatient hospitalization, a multidisciplinary approach produces the best outcome at the time of discharge. Older adolescents and patients with traumatic leg amputations have longer stay during initial hospitalization and higher hospitalization rates (46). A single limb is involved in more than 90% of acquired amputations, of which 60% involves the leg (see Table 13.2). The male-to-female ratio of acquired amputation is 3:1. TABLE 13.2 LONGITUDINAL LIMB DEFICIENCIES Tumors are the most frequent cause of amputations due to disease. Tumors represent the most common cause of amputations in the European Surveillance of Congenital Anomalies (EUROCAT) data system (1). The highest incidence of malignancy is in the 12- to 21-year-old age group. Osteogenic sarcoma, Ewing’s sarcoma, and the rare rhabdomyosarcoma are responsible for the majority of tumors resulting in amputation (47,48). Unprecedented improvement in survival has occurred with earlier detection and combined therapy (49). Definitive surgery for osteosarcoma depends upon the site of the primary tumor and the extent of invasion or metastasis (50). Surgical removal of the affected bone and the surrounding soft tissue remains the treatment of choice, whether by amputation or limb-salvage procedure. Limb salvage with an endoprosthesis can be offered to 90% of children with osteosarcoma (49–51). This procedure, which involves replacing the affected bone with a metal endoprosthesis, is accompanied by orders to prohibit contact sports. With the advent of extendable endoprostheses, it has been suggested that children who have undergone this treatment have results that are superior to those who have undergone amputation surgery (52–54). Families may wish to pursue this due to the improved cosmesis, to prevent the loss of a limb in a growing child, and ultimately, to achieve the best functional outcome. The surgical procedure of choice is that which obtains a tumor-free margin of 5 to 8 cm above the proximal limit of the medullary tumor. The decision to proceed with limb salvage or amputation is dependent on the aggressiveness of the tumor, the stage, the responsiveness to neoadjuvant therapy, and the likelihood of obtaining tumor-free margins (55–57). The knee poses a challenge for soft tissue sarcomas. Despite complications, the knee may be reconstructed with allografts (58,59). Rehabilitation physicians discuss the likely functional outcomes of each choice and provide continued support and maintenance of functional status during the chosen course of treatment. During the limb salvage, amputation, and recovery process, rehabilitation physicians and therapists promote strategies to prevent any decline in function, treat pain, and anticipate progression of potential treatments. Chemotherapy has now proven to be an effective adjunct to surgery. Prior to 1972, only 15% of the children were disease-free and survived with surgery, compared to the 60% to 70% who now survive with surgery and the addition of chemotherapy (60,61). Rehabilitation may be confounded by factors of fluctuation in limb-volume status, fatigue, and the psychological aspects of combined treatments. Physical therapy emphasizing range of motion (ROM), strengthening, and functional activities is important for children with lower extremity sarcoma after limb-salvage surgery (62). Outcomes were similar for ambulation, stair climbing, employment, and psychological adjustment when comparing amputation to limb salvage as surgical management of sarcomas. Patients benefit functionally from gait training with prosthetic devices following amputation (63,64). Additionally, osteosarcoma patients who underwent amputation compared to limb salvage experienced similar socioeconomic outcomes, such as education, employment, and marital status at a 20 year follow-up; however, the most important factor for determining the quality of life in these patients was the functionality of the limb regardless of amputation or limb-salvage procedure (65,66). Conflicting studies argue that limb-salvage procedures appear to improve quality of life over amputation; however, this study did not directly evaluate functional status (67). INFECTIONS Infectious emboli from meningococcemia may autoamputate limbs or digits (68). The process frequently involves all four limbs. Growth plates may be affected, resulting in angular deformity and the need for surgical epiphysiodesis (69). Frequently, the skin is affected as well as the limb (70). Multiple surgical skin grafts limit the prosthetic fitting; a coordinated burn team is often best prepared to handle initial management (71). Over the past few decades, the incidence of invasive meningococcal disease in the United States has remained relatively stable (72–74). Pneumococcal septicemia also can produce purpura fulminans, characterized by acute onset of rapidly progressive hemorrhagic necrosis of the skin and thrombosis (29). An example of the multiple distal amputations and angular deformities caused by emboli from infections is seen in Figure 13.4. SURGICAL APPROACH: GENERAL PRINCIPLES Adherence to the general principles of childhood amputation surgery promotes optimal function. The principles are: (a) preserve length, (b) preserve growth plates, (c) perform disarticulation rather than transosseous amputation, (d) preserve the knee joint whenever possible, and (e) stabilize and normalize proximal portions of the limb (75). The cardinal surgical dictum to conserve the whole limb length if possible is true for children as well as adults. In growing children who require amputation, disarticulation rather than a transdiaphyseal amputation may be preferred (76). Disarticulation preserves the epiphyseal growth plates and ensures longitudinal growth (77). Disarticulation also avoids the development of terminal or appositional overgrowth of new bone. Frequently, children with limb differences experience problems with the length of their affected limbs. Because of known growth patterns, limb-length differences tend to vary with patient age and involved bone, and growth projections over time are crucial for surgical decision making. For example, 70% of femoral growth occurs at the distal physis (78), while the contribution of the proximal tibial growth plate is about 57% of the total length. So saving a short tibial remnant in a 4-year old reaps greater biomechanical benefit than for a 17-year old, despite the risk of bony overgrowth. In the younger child, saving the knee is more meaningful, with potential for better prosthetic function (79). Figure 13.5 demonstrates a very short salvaged tibial remnant successfully managed with below-knee prosthetic intervention. Fortunately in the pediatric population, tissue viability is enhanced, so strategies such as split thickness skin grafting, skin traction with delayed closure, and wounds closed over more tension heal better compared to the same techniques in adults, providing viable wound coverage and incision closure. Figure 13.6 demonstrates extensive skin and tissue loss, with successful subsequent healing and prosthetic tolerance. FIGURE 13.4 Angulated amputations as a result of meningococcemia and subsequent purpura fulminans. Terminal overgrowth, often referred to as spiking, at the transected end of a long bone is the most common complication following amputation in the immature child (80,81). Diaphyseal overgrowth may also occur in children with acquired intrauterine anomalies, such as amniotic band syndrome, in which the epiphysis is no longer present. It occurs most frequently in the humerus, fibula, tibia, and femur. During appositional growth, the distal bone begins to form in the shape of an icicle. Figure 13.7 demonstrates a typical radiographic finding of terminal overgrowth. As the pointed segment creates insult to the adjacent soft tissue, a bursa may form to protect the distal residuum. During this time, the child may experience significant pain with reduced tolerance of prosthetic use. Frequent socket modifications are necessary to accommodate these anatomic changes. Treatments such as aspiration, steroid injections, and stump wrapping are usually ineffective. Unfortunately, the rate of growth may be so vigorous that the bone pierces the skin; at this stage, the treatment of choice is surgical revision. Distal resection and stump capping with the use of autografts or plastic polymers are surgical options (82). Once surgery becomes necessary, the problem is likely to recur until skeletal maturity. Each time that bone is resected, the overall length of the bone is reduced, thereby reducing its mechanical advantage for control of the prosthesis. Bone spurs may form at the periphery of the transected bone, and resection may be necessary. The resulting stump scarring, which interferes with weight-bearing, requires prosthetic modifications and exploration of custom interfaces. Plastic surgeons are involved with reconstruction of skin flaps or with complicated repairs of residual limbs (51,83). In Figure 13.8 an example of complicated residual scarring is shown. FIGURE 13.5 Surgical differences between pediatrics and adults enable this very short salvaged tibial remnant to be successfully managed with below-knee prosthetic intervention. FIGURE 13.6 The photos for Figures 13.5 and 13.6 show the surgical difference between pediatric and adult acquired limb deficiency. Skin grafting and the ability for healing allows surgeons to be more creative with wound coverage. Despite extensive scarring, prosthetic fitting can still occur. FIGURE 13.7 Terminal overgrowth represents a common problem in the limb deficient child. The fibula continued to grow in length in this radiograph representing problems with skin integrity and prosthetic fitting. (Photo courtesy of the personal collection of Dr. Vincent Mosca, MD.) FIGURE 13.8 Residual limb with reconstructed skin grafts and custom liner. PHANTOM SENSATION Phantom sensation is an individual’s sensation of feeling a missing limb like it were actually present. These sensations are rarely reported as painful or unpleasant in the pediatric population. Since phantom sensation is not painful, no treatment is necessary; however, if phantom limb pain becomes an issue in older children and adolescents, it can interfere with the rehabilitation process. Phantom limb pain rarely occurs in children under 10 or during growth, but is reported in teenagers. In addition, children with congenital limb deficiencies are less likely to experience phantom sensations than those with acquired amputations, but it is important to recognize that it can occur (84,85). Phantom sensations in children with limb deficiency are explainable if we recognize the brain as a generator of sensory information (86). UPPER LIMB There are differences in the approach, acceptance, and management of the upper limb amputee versus the lower extremity amputee. Despite significant and ongoing improvements, upper limb prosthetic devices do not yet replace the sensory function of the hand, and are best considered as a mechanical tool (87). The hand is used to explore the environment and to manipulate objects within it. The hand needs to reach the body and precisely approach an object, grasp, and then release it. Acceptance of the prosthesis is variable (88). Frequently, the exposed skin of a residual limb is preferable to an encased limb. Stump sensation may even be enhanced to compensate for the loss of prehensile area (89). In ipsilateral congenital limb deficiencies, issues with organogenesis can occur and may require further evaluation; however, bilateral limb deficiencies may be associated with craniofacial abnormalities (8). Scoliosis occurs frequently in this population, but rarely requires surgical correction (90). COMMON UPPER LIMB DEFICIENCIES Digital Deficiencies Digital deficiencies are common but rarely present in isolation. Removal of additional digits and intervention with Z-plasty procedures produce acceptable results for children with polydactyly and syndactyly, respectively. Amniotic band syndrome or Streeter’s dysplasia commonly presents with digital constriction banding, though the etiology of this condition remains controversial. In addition, other anomalies may be present, ranging from evidence of other banding, to craniofacial clefting (in 78%) or abdominal to one or more lower limb amputations (in 70%), and abdominal wall, spine, or thorax (in 52%) that have occurred in utero (91). Hand impairments can be attended to if they affect the child’s ability to perform activities of daily living (ADLs) or don and doff a lower extremity prosthesis (92). Etiologies such as Moebius syndrome and Poland syndrome (sequence) result in digital deformities associated with a more serious underlying condition. Moebius syndrome often affects the sixth and seventh cranial nerves, which compromises the child’s ability to visually follow objects, swallow, and communicate. In addition to hand anomalies, Poland syndrome involves a partial absence of the ipsilateral pectoralis muscle and hypoplastic chest; therefore, a more proximal evaluation of any distal absence of limb is indicated. Absence of individual digits creates a multitude of surgical and nonsurgical options. These include no intervention, therapy to enhance hand function, pollicization, or toe transfers. Due to the physiologic function of the normal thumb, hand impairments can vary widely, depending upon which digit(s) is/are absent. There is often greater consideration for surgery and function if the thumb is absent. Pollicization to the most radial digit can provide oppositional grasp (93). Through this microsurgery procedure, children can achieve remarkable functional improvement with improved cosmesis (94). Toe transfers can be transplanted from the second or third ray and minimize effects on gait mechanics (95,96). Partial Hand and Wrist Disarticulation Deficiencies Partial hand deficiencies are quite common and are often treated as wrist disarticulation level limbs. Very small underdeveloped vestigial digits, sometimes referred to as nubbins, are present in a majority of these cases, as is shortening of the ipsilateral radius and ulna. These vestigial remnants are rarely problematic, nor do they need to be surgically removed. The child can be quite functional with no intervention, and these remnants may enhance tactile input. The major functional drawback of this particular limb length is the inability to perform prehensile tasks with the involved limb, though the adaptability of the child can be remarkable. Plastic surgeons may be consulted for digit- and hand-level deformity. Transverse Deficiencies of the Forearm Transverse deficiency of the upper third of the forearm is the most common (major) upper limb deficiency (97). The clinical presentation of these children is similar to that of children with longer, transradial residual limbs with ipsilateral humeral shortening and small nubbins. The proximal radius in these shorter residua is often unstable, subluxing anteriorly during full extension, giving the appearance of hyperextension of the elbow. This creates a challenge to prosthetic fitting because the amount of length available for contact with the prosthesis is limited. Residual limbs spanning to the middle third or longer, tend to be more easily fit with a prosthesis, as they have more surface area over which to distribute the forces of the socket interface. They also have longer lever arms with which the patient can control the prosthesis. The ease of use of a prosthesis contributes to more functional tasks and prosthetic training. Traditionally, surgical intervention at this level remains rare. However, more recently, some pediatric hospitals have implemented pediatric hand transplant programs. These are generally in the research stage and remain limited to older pediatric patients due to size and logistics issues (98). If prosthetic intervention is not attempted or accepted, bimanual tasks will be performed via grasping of objects in the cubital fold, between one’s legs, in the axilla region, or under the chin. Both with and without prosthetic intervention, children with this level of congenital amputation can expect full functional independence. While this revelation casts some skepticism on the objective value of prosthetic intervention, technology continues to improve the functional enhancement afforded by prosthetic intervention. Elbow Disarticulation and Transhumeral Deficiencies The more articulations that are absent, the greater the functional deficit. When the elbow joint is compromised or absent, the child has fewer options to assist in pre-positioning the distal limb in space and experience more limited pronation and supination for functional tasks of the prosthesis. In this case, the child relies solely on the muscles and ROM of the shoulder complex. The true elbow disarticulation limb has the distal epiphysis present, which is important for the overall growth of the residuum. A drawback of any disarticulation is the lack of room to fit prosthetic components and maintain humeral length equality by implementing elbow joints at the natural level. Transverse deficiencies of the humerus are analogous to acquired transhumeral amputations in children. The residual limbs are often medium to short in length compared to their contralateral limbs. This level of deficiency has been previously noted as the most common to experience diaphyseal overgrowth. This leads to a shorter limb that potentially is less functional and amendable to prosthetic fitting. Shoulder Disarticulation and Intrascapulothoracic Deficiencies It becomes increasingly difficult to restore the functions of the anatomic arm as the level of deficiency reaches the shoulder and higher with current technology, though promising innovations for prosthetic restoration are on the horizon. Children with remnant humeri can use these segments to assist in their activities. Often, the axilla will be used to assist these individuals to grasp and manipulate objects. If the child has unilateral limb deficiency, the contralateral noninvolved limb will be or become the dominant side for grasping, with manipulation taking place between the knees, in the mouth, or trapped between chin and chest or chin and shoulder. When the child has bilateral deficiencies at the shoulder level, the latter method is all that is possible to grasp objects. In these cases, the child will be strongly encouraged to use the feet to grasp and manipulate objects, often with remarkable facility. Many designs of upper extremity prostheses require a degree of body movement (excursion) to operate the mechanical components. Most of this excursion is not present in the shoulder disarticulation level, as glenohumeral flexion no longer exists as a source of control input. This is further magnified when children have an intrascapulothoracic (forequarter) level of involvement, as they only have uniscapular motion to capture for prosthetic limb control. These two issues will be discussed at length in the following sections. UNCOMMON UPPER LIMB DEFICIENCIES Longitudinal Deficiencies of the Forearm Radial deficiencies are approximately three times as common as ulnar deficiencies, occurring in 1 in 30,000 and 1 in 100,000 live births, respectively (99). Fanconi anemia; thrombocytopenia and absent radius (TAR); Holt–Oram syndrome; vertebral defects, anal atresia, tracheoesophageal fistula, radial and renal dysplasia (VATER); and Robert’s syndrome are just a few examples of etiologies with associated radial involvement with variable level of limb deficiency, contractures of joints, and syndactyly (100,101). Figure 13.9 illustrates the complex issues with Robert’s syndrome. The clinical presentation of radial deficiencies usually involves the radial-side digits of the hand as well. Depending upon the classification of the radial deficiency, prehensile capabilities may be compromised by a hypoplastic or absent thumb. In these situations, pollicization or toe-transfer procedures are considered. Treatment for radial deficiencies is focused on reconstructing the thumb and, in both the radial and ulnar deficiencies, is directed at centralization of the hand (102). Ulnar deficiencies are associated more with musculoskeletal conditions than systemic conditions, and isolated genetic predispositions have been discovered (13). Cornelia de Lange syndrome, ulnar mammary syndrome, and ulnar fibula dysplasia are examples of syndromes that involve ulnar deficiencies. With ulnar-side involvement, the thumb and another digit are usually present. Central ray syndrome, a form of ectrodactyly, had been described previously as having genetic predisposition. This is commonly referred to as “lobster claw,” as the central component of the hand and/or feet are absent. This can present as a mild condition, with the more ulnar and radial digits still present, or it can present as two longer and thicker digits. Functional abilities with this condition will vary, depending upon the degree at which the syndrome affects the limb. Many of these individuals will not need prosthetic restoration, as the limbs are at full length and have prehensile and tactile capabilities. Surgical reconstruction may be recommended if the child lacks the oppositional capabilities that the thumb usually offers. FIGURE 13.9 Child with Robert’s syndrome. Note flexion contractures of all limbs. Longitudinal Deficiencies of the Humerus When a longitudinal deficiency of the humerus is present, it is often associated with deficiencies in the radius and ulna and with phocomelic digits. The length of the arm is compromised, which reduces the envelope of space needed to perform some bimanual tasks. For this reason, creative prosthetic fitting is more likely a consideration compared with isolated longitudinal deficiencies of the forearm. The shoulder complex may be compromised as well. Therefore, with prosthetic fitting, the child would most likely require some externally powered components. Frequently, the phocomelic digits will be used to provide manipulation of these components. INTERVENTION, PROSTHETIC TREATMENT, AND ADAPTIVE EQUIPMENT Although prosthetic treatment may seem indisputable for an individual with a limb absence or acquired amputation, the decision is not as straightforward as one might imagine. The inability to provide or restore the function of the human arm and hand poses great challenges to individuals with partial or complete limb loss (103). These fittings are generally limb-level-dependent as well and vary among oppositional, body-powered, and externally powered options. Discussing what the patient and family value as well as goals for a prosthesis prior to ordering may help with expectations and limit rejection. Acceptance of prosthesis is a complex issue; factors that influence acceptance include level of limb loss, presence of other complicating medical conditions, comfort and usefulness of the prosthesis, and acceptance of the limb deficiency by the family. In general, the higher the limb absence, the less likely it is that a child will find a prosthesis useful enough to wear it regularly. For example, transradial patients will tend to wear their prostheses more than transhumeral patients, and transhumeral patients will tend to wear their limb more than shoulder disarticulation patients (90). In general, for upper extremity loss, prostheses can be considered tools to accomplish certain tasks rather than being a necessity for routine day-to-day activities. Goals of early intervention and training revolve around achieving age-appropriate milestones. Children with upper limb differences frequently achieve developmental milestones at or around the same age as children without limb anomalies. Prostheses are generally considered around 3 to 6 months of age (104). Until recently, 6 months of age used to be the time at which fitting was initiated (105,106). This was the age chosen because it was the time the child was expected to have achieved sitting balance and to begin to engage in bimanual tasks. Clinical experience versus evidence-based study guides fitting timetables (107). Although there are general guidelines for fittings, the initial fitting is something that is discussed in the clinic between the team members and family. Many children will be fitted with prostheses prior to 1 year of age. Early prosthetic fitting is designed to encourage bimanual tasks, establish a wearing pattern, increase overall independence, provide for symmetrical crawling, and reduce “stump dependence”—sensory dependence on the end of the residual limb (107). Early fitting does not guarantee acceptance (108, 109). The prostheses needs to fit comfortably, which can be challenging to assess in an infant, be relatively easily donned, equalize lengths with the noninvolved limb, allow for growth, and provide restoration acceptable to family (82). Several different terminal devices may be considered for the first prosthesis. Age-appropriate prostheses are fitted to children; oppositional prostheses are generally the first design utilized. Options include hands, hooks of various shapes, mitts, and other nonhand designs. The vast majority of parents prefer a terminal device that looks like a hand. For this reason, it is recommended that a passive hand be provided rather than a hook or other nonhand device. The two basic passive hand options for infants are the closed, “crawling hand” design and the open hand design, or a hybrid between the two. The parents should be involved in the decision-making process—this involves providing information about the pros and cons of each style and, more importantly, letting the parents decide which design is most acceptable in their eyes. If parents view prosthetic use as a benefit, as the child ages both the patient and family may be more inclined to evaluate prosthetic components based on functional qualities in addition to appearance. If parents are involved in the decision and accept the device, they are more likely to encourage the youngster to wear the prosthesis. Figure 13.10 shows an infant passive hand. FIGURE 13.10 RSL Steeper infant foam-filled passive hand. FIGURE 13.11 Transcarpal limb deficiency with adequate length for bimanual tasks. It is questionable whether it is appropriate to fit children with partial hand deficiencies and wrist disarticulations at a very young age. They have long residua and can use them for bimanual tasks. Figure 13.11 illustrates a transcarpal limb deficiency with adequate length for function. The prosthesis would serve the purpose of providing a wearing pattern and also reducing dependence on the sensation of the limb. The latter can arguably be considered as a positive rather than a negative. Opposition posts are sometimes considered for the child with carpals and wrist motion. These devices can be rigidly fixed or placed in several different positions to accommodate for grasping different-sized objects. For the child with a limb that extends distal to the elbow, the initial prosthesis is usually self-suspending, using a supracondylar design, with or without a suspension sleeve. If this is not achievable, a narrow Dacron harness may be designed in a figure-eight or figure-nine configuration. This harness should be easy to put on the child, have elastic as part of the straps for increased shoulder motion, and have snaps or fasteners that make it easy to put on and take off, ideally by the child without the need for assistance by a parent. The same oppositional terminal device options are appropriate for the child with a limb deficiency proximal to the elbow. The major difference between these levels is that the absence of the elbow joint makes it more difficult to pre-position the terminal device for bimanual tasks. The child is not cognitively ready for an articulating elbow; therefore, a curve-shaped “banana” arm is often provided in order for this child to engage the prosthesis with the contralateral hand as well as reach levels that are closer to the midline and face (110). Figure 13.12 displays the passive “banana arm” prosthesis. The next developmental milestone is walking, which usually occurs at 11 to 13 months of age. This will indicate that the child is ready for a more sophisticated upper extremity prosthesis. At this time, the child is ready to perform simple grasp-and-release activities using the prosthesis. It is imperative that the family be involved in the clinical decision making about their child’s prosthesis. The prosthetist should design the prosthesis in a manner to accommodate growth. It is best to keep the control system as simple as possible at this early age in order to ensure early success. Other developmental factors to be considered are understanding of holding function, attention span longer than 5 minutes, and willingness to be handled by an occupational therapist to go through terminal device opening motion. The prosthetic training at this stage relies on a child’s ability to grasp a cause-and-effect-type relationship when participating in therapy. When the child is developmentally ready for terminal device activation, options include body-powered hooks or hands as well as myoelectrically controlled hands. The majority of parents prefer hands over hooks due to the cosmetic improvement gained; the hands that provide optimal function at this age are myoelectrically controlled, though the weight of the prosthesis must be considered relative to the length of the residual limb. At this age, the simplicity of control is of paramount importance. An electric hand that is controlled by one electrode in a voluntary-opening control scheme has proven effective and natural. This electronic scheme permits the child to activate the hand opening with a contraction (usually on the side of the wrist extensors) and relaxation that enables the hand to automatically close, or “cookie crusher” design. This electromechanical design is analogous to a split hook, voluntary-opening prosthesis. Designing such an electronic control scheme eliminates the need for the child to maintain muscle contraction in order to continue grasping the object, which proves tiresome and may limit function. As the child grows older, another electrode can be added to the flexor side of the forearm, enabling the child to have volitional control opening and closing the myoelectric hand in a more physiologic manner (111). FIGURE 13.12 Transhumeral passive “banana arm” prosthesis. Myoelectric hands of the past were too large and difficult for a 1- or 2-year-old child to use successfully. Therefore, it was recommended that these hands not be fitted on children until 4 to 5 years of age. Currently because of improvements in prosthetic design, it is common for these hands to be fitted successfully on 1-year-old children. Prosthetic technology has improved dramatically as a result of miniaturization, improved materials, battery weight, and simpler control to better meet the needs of very young children. Figure 13.13 shows a transradial myoelectric prosthesis with myoelectric hand terminal device. Body-powered devices may not work well for this age group because they lack the requisite force and excursion, as well as the cognitive ability, to relate shoulder motions to terminal device operation. The voluntary-opening-style terminal devices permit the user to grasp an object and allow the force of the elastic bands or springs to keep the object in the terminal device. As the number of bands increases, the amount of force required to open the terminal device increases as well; thus, the child may not be able to overcome the force required to activate the terminal device. The designs of voluntary-opening terminal devices for children are not very aesthetically pleasing, with the exception of the mechanical hands. The hands, however, have the drawback of providing minimal efficiency. Once a cosmetic glove is applied to the mechanical hand, it can lose up to 40% of its efficiency, compared to the function of the hand without the glove. Voluntary-closing terminal devices have gained in popularity, although the child must maintain force and excursion through the harness to maintain grasp on an object. The amount of grasping force is directly proportional to the force that the child puts into the harness (112). The prosthetic team can predict the ability of the child to control the myoelectric components when using evaluative tools such as the Capacity of Myoelectric Control (113,114), by means of developmentally engaging devices triggered by the muscles targeted for prosthetic activation. FIGURE 13.13 Transradial myoelectric prosthesis. By the time children are 4 or 5 years old, they are able to operate virtually all types of prosthetic components and control schemes presently available (112). The developmental milestones described previously should guide the fitting schedule of the child with transhumeral limb involvement. Because of the nature of a transhumeral prosthesis, it can be more of an encumbrance than the transradial design. This can cause difficulty in rolling over, and may impede the child’s development if fitted too early. The terminal device should be activated shortly after the child begins to walk. Terminal devices for the transhumeral level are the same as for the transradial. The addition of a prosthetic elbow is the key difference. The first prehensile prosthesis will employ a friction elbow to allow positioning of the terminal device. It is useful to limit the ROM at the elbow by producing flexion and extension stops to prevent the elbow from flexing excessively during weight-bearing activities (eg, crawling). The initial prosthesis may be suspended by a harness or by silicone suction suspension. The silicone suction socket (3S) has proven effective because it allows free ROM at the shoulder and provides excellent suspension. The child with a transhumeral deficit should be fitted with an activated terminal device once he or she begins to walk. Considerations for terminal device selection include appearance, weight, ease of operation, and cost. With the initial prosthesis, the myoelectric hand offers reasonable appearance and ease of operation when controlled by a single-site voluntary-opening circuit; however, it is a heavier and more expensive prosthesis compared to body-powered. By starting with a passive hand device, a child may demonstrate ability to tolerate a prosthesis prior to incorporating more expensive myoelectric components. Either voluntary-opening or voluntary-closing designs can be used successfully by the child with transhumeral limb involvement once the child has sufficient strength and the cognitive ability to understand how to operate the device. This usually is possible at 2 to 3 years of age. When the child is strong enough to operate an active elbow, usually at ages 4 to 5, a conventional body-powered elbow may be provided; however, locking of the elbow by conventional methods may prove challenging. Depending on the length of the residual limb, the child may have insufficient strength/excursion to operate the body-powered elbow. In this case, an electric elbow may be considered, although the increased weight may preclude this option. The terminal device illustrated in Figure 13.14 is a voluntary-opening split hook and can be utilized on both transhumeral and transradial deficiencies. The shoulder disarticulation level is treated differently due to the challenge in positioning the shoulder, elbow, and terminal device. With bilateral involvement, different components may be selected for each side, with function and gadget tolerance guiding prosthetic decision making. The child may be fitted with a passive endoskeletal shoulder and elbow with an active terminal device. Externally powered hands controlled by either electromechanical rocker switches or force-sensing resistors may be used. The child is encouraged to maintain good ROM for shoulder elevation/depression and protraction/retraction in order to make contact with these input devices. The return or enhancement of function using these devices is quite limited. Therefore, there are no “right” philosophies for the fitting of these complicated cases. The team should recognize that prostheses need to be useful to the child, or they will be rejected despite careful decision making. A task-specific prosthetic terminal device is illustrated in Figure 13.15. The patient with bilateral total upper extremity transverse deficiency and phocomelic residua rarely requires amputation revision; indeed, the terminal digits can activate switches or myoelectric sensors (103). In the case of higher level bilateral deficiencies, it is wise to start as simple as possible, recognizing that each child has a certain level of tolerance for “gadgets.” With the vast array of prosthetic components now available, it would be easy for the well-intentioned clinic team to recommend components that would overwhelm the user and lead to prosthetic rejection. Critical factors in the success of the high-level bilateral amputees are prosthetic weight, complexity of control, proprioceptive feedback, wearing comfort components, and motivation and attitude of child and family.



Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree