4 Clinical Application of Maggots
The Classical Free-Range Maggot Dressing System
The edges of the wound should be protected from the maggots’ digestive enzymes, from the infectious, necrotic wound drainage, and from the tickling or itching sensation caused by the maggots crawling over normally innervated skin. This is best done by covering the wound edges with strips of self-adhesive hydrocolloid, or cutting a hole the size of the wound in a sheet of hydrocolloid before placing it over the wound (Sherman, 1997). Zinc paste can then be applied to remaining areas of exposed skin to avoid irritation.
The next step is to place the young, 2-4-mm-long larvae on the wound. The recommended dose is 5 to 8 larvae per cm2 of wound surface area. The maggots can be rinsed out of the container onto a piece of nylon net, using saline solution, and then transferred from the net to the wound site either by using closed forceps or a swab, or simply by inverting the net over the wound site. Some manufacturers supply the maggots already on sterile gauze which can simply be removed from the vial, cut to size, and placed on the wound. Nylon netting, Dacron chiffon or other similar fine porous material is then glued and/or taped on top, completing the “primary” cage-like dressing. Refrigerating the maggots beforehand will slow them down, else they may escape from the wound as fast as you put them there.

Fig. 16 A container of first to second instar larvae ready for use in therapy.

Fig. 17 The classical free-range maggot dressing.
The last step is to cover the primary dressing with a simple light gauze bandage (“secondary dressing”), which functions to absorb exudate and liquefied necrotic tissue during the treatment period. The secondary dressing can be readily changed as needed without letting the maggots escape. Moreover, the activity of the maggots can be observed through the translucent cage dressing whenever the secondary gauze dressing is removed.
The maggots are left in place for 2 to 4 days, after which they can be removed from the wound by peeling back the primary dressing while wiping up the maggots with a wet gauze pad, sandwiching the maggots between the dressings and the gauze pad. Alternatively, the maggots may be irrigated from the wound. The dressings should be discarded as soiled infectious waste (“redbag waste”), making sure to seal the bag containing the maggots in order to prevent their escape while they await autoclaving or incineration
The Containment-Bag Maggot Dressing System (Biobag)
Allowing maggots to crawl freely on the wound surface is not optimal for some wounds. Sometimes it is preferable to prevent the maggots from roaming on fragile tissue, or into a deep cavity (i. e., the pleural space or the nasopharynx) just beneath the necrotic wound. Furthermore, direct exposure to the maggots’ sharp mouth hooks and spicules can be painful for some patients.
Also, it takes more time, effort, and training to properly dress a wound using free-range maggots as opposed to maggots confined in bags. In light of these considerations, some maggot therapists prefer maggot-containment dressings for delivering maggot debridement therapy. Contained maggots have been shown to be an effective method of maggot debridement therapy (Grassberger & Fleischmann, 2002). Two major designs are being used, both of which completely enclose the maggots. The Biobag (Polymedics, Belgium) is composed of polyvinylalcohol, an open-cell polymer; disinfected maggots are heat-sealed within this four-sided pouch. Maggot secretions flow freely out of the Biobag and into the wound; the Biobag absorbs the liquified necrotic wound drainage, nourishing the maggots within. Polyvinylalcohol is also associated with wound cleansing and healing (Mutschier et al., 1980). Some containment systems are composed of nylon or Dacron chiffon. These sheer fabrics allow free passage of the maggot secretions and wound drainage, and also allow the maggots within to come into limited direct physical contact with the wound. Unlike polyvinylalcohol, however, these fabrics do not actively absorb wound drainage nor are they themselves associated with wound healing. Both containment designs offer the advantages of easy application and removal, decreased discomfort, decreased opportunity for maggots to escape, and the esthetic satisfaction of not having the maggots “loose” on the wound.

Fig. 18 Preparation of a Biobag.
It has been argued that maggot containment systems, by limiting or preventing direct contact between the maggot and the wound, are less effective and efficient than maggot debridement therapy with free-range maggots (Thomas et al, 2002) because the host does not have the mechanical benefit of the maggots active mouth hooks and spicules. A clinical trial comparing the different containment systems with free-range maggot therapy clearly is needed to better evaluate and compare these different approaches.

Fig. 19 Schematic view of the Biobag system. Absorbed fluids consist of liquefied necrosis, pus, bacteria and wound exsudate.
Indications for Maggot Debridement Therapy
The treatment of acute wounds is straightforward, and fundamentally has been the same for thousands of years: stop the bleeding, and then protect the wound from harmful micro-organisms and environmental hazards. Usually, the body will heal itself quite nicely under these conditions.
When the body’s ability to heal the wound is impaired, or under adverse environmental conditions, then the wound may become chronic. Chronic wounds put the patient at continued risk of infection, which may impair the body’s capacity to heal even further. Chronic wounds result from, and may themselves cause, further imbalances in the biochemical mediators (cytokines) that regulate and promote wound healing.
The Diabetic Wound
Three common errors, individually or collectively, frequently lead down the fateful path to amputation: diabetes management, lack of diligence on the part of the patient in monitoring and adjusting the blood glucose, and negligence in the imperative, meticulous examination of the feet. Both physician and patient must examine the feet at every opportunity. Most amputations in diabetic patients could be avoided by local preventive measures, such as wearing comfortable orthopedic shoes, the prevention or immediate medical treatment of all injuries, and surgical correction of skeletal foot anomalies to improve the distribution of pressure across the foot.
Chronic non-healing foot wounds in diabetic patients are characterized by an absence of natural wound healing processes. Progressive tissue death tends to occur, resulting in the loss of toes (diabetic gangrene) or the destruction of skin on the soles of the feet and in the underlying layers of padding tissue (malum perforans). Dead tissue immediately breeds bacteria and fungi. The breakdown of proteins leads to the characteristic stench of necrotic tissue. Microbial invasion of the neighboring healthy tissue may lead to further necrosis, cellulitis, lymphangitis, bacteremia, osteomyelitis, or death.
Many diabetic patients with foot wounds are ideal candidates for maggot debridement therapy (Mumcuoglu et al., 1998; Fleischmann et al., 1999; Sherman, 2002). The maggots gently and thoroughly remove necrotic tissue by mechanical action and by enzymatic liquifaction (proteolytic digestion). The maggots secrete antimicrobial peptides into the wound, kill ingested bacteria in their gut, and alkalinize the wound. Furthermore, the increased production of wound secretions rinses bacteria and bacterial toxins out of the wound. Maggot secretions promote wound healing and often dramatically shorten the wound healing time, provided that blood flow in the major arteries is adequate to support healing.
Comments:
If maggot debridement therapy did such a good job healing this wound, thus preventing amputation, imagine how much sooner it would have healed, and how much less destruction there would have been, if this patient had been treated with maggot debridement therapy earlier in the course of his non-healing wound!

Fig. 22 Same patient, final result after wound healing.
Chronic Leg Ulcers
Chronic ulcers of the lower leg can develop for a number of reasons. The most common cause is impaired blood flow, for example an insufficient arterial blood supply to the limb or failure of venous return to the heart. Mixed ulcers have both arterial and venous components.
The etiologic diagnosis of chronic leg ulcers is difficult and often costly. However, specific therapy requires an exact diagnosis. If the arterial supply to the limb is deficient, the goal of treatment must be to improve the flow of the blood and to restore arterial patency. If compromised venous return has led to vascular congestion, leg compression (compression bandages or elastic stockings) must be carried out, augmented by surgical measures as needed.
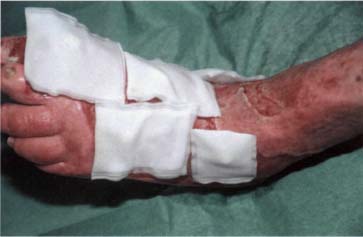
Fig. 23 Biobags applied to foot wounds.
Maggot therapy may help heal the leg ulcer, but it will not resolve the underlying cause. Maggot debridement therapy should be performed only after the underlying circulatory disorder has been properly diagnosed and treated. Malignant neoplasms can arise from non-healing wounds that persist for several years. Therefore, it is imperative to examine the histology of tissue samples taken from the edge of the wound. Current findings indicate that medicinal maggots do not have a lethal effect on neoplastic tissue. Immediate surgical or dermatological treatment of tumors is therefore required.
A good knowledge of pathophysiology is required for successful treatment of non-healing leg ulcers that have persisted for many years. The process of wound healing is normally very effective and usually results in complete closure without any specific intervention. The acute response is hemostasis; the next step is the prevention of infection. By producing large quantities of wound fluids, bacteria and bacterial toxins are rinsed out of the wound, preventing them from colonizing the wound surface. Cellular and humoral immunity further protect the body from microbes. Within a few days, an extremely resilient layer of pink, vascularized granulation tissue develops, sealing and protecting the wound from harmful micro-organisms. The production of wound fluid now decreases, and new skin can be produced around the wound edges to achieve wound healing. Collagen is laid down, the wound contracts, and the edges are brought closer together as the skin regenerates. These phases do not occur in stepwise sequence, but rather as a continuum.
Serious consequences can arise from the prolonged failure of wound healing. Friable granulation tissue or poorly vascularized scar tissue may develop over the wound bed, blocking the absorption of essential nutrients from layers of healthy tissue below the wound, or preventing new skin growth or skin grafting.
Maggots cannot easily dissolve tendons, ligaments, bone, or large plates of scar tissue. Maggots fail at this task whereas a surgeon can eliminate the problem very quickly, easily, and inexpensively using a scalpel.
In spite of this limitation, the adjuvant use of maggots for treatment of leg ulcers can produce outstanding results, provided the maggots are assigned their specific niche in the overall treatment strategy and provided they are selectively employed to debride wounds and combat infections.
Maggots have been reported to promote some Pseudomonas and Proteus wound infections. Organisms in the wound should therefore be regularly isolated and identified. If evidence of a Pseudomonas or Proteus infection is found, the patient may need to be switched, at least temporarily, to another antimicrobial treatment method.









