Abstract
Osteoporosis is a common complication in children with motor impairments. They have a higher risk of fractures (20% during their lifetime) mostly at femoral level. Furthermore, these children have pain yet no clear relation has been established between osteoporosis and pain. The efficacy of bisphosphonates has been validated in adults and for children with osteogenesis imperfecta (OI). However, its use in children with motor impairments has not yet been validated.
Patients and methods
Retrospective study on the medical charts of children presenting neurological diseases and motor impairments associated to secondary symptomatic osteoporosis. These children underwent treatment with Pamidronate ® intravenous infusions (I.V.) in Lyon and Valence between 2002 and 2008. Data were collected on pain control, incidence and frequency of fractures and bone mass density (BMD).
Data on adverse events were also collected to evaluate treatment’s tolerance.
Results
Twelve children’s charts were studied for a total of 50 Pamidronate ® I.V. infusions. Regarding treatment’s efficacy, we observed a clear decrease and even total relief of the pain with improvement reported after 98% of perfusions. Regarding BMD, there was a real improvement after the treatment (e.g., lumbar BMD measures, −46.5% before treatment and −27% after treatment). The adverse events, flu-like syndrome, muscle pain and asymptomatic hypocalcemia, were minor and quickly reversible.
Conclusion
It seems quite essential to screen for osteoporosis-related pain in these children and treat them quickly to avoid a negative impact on their quality of life. Treatment with I.V. bisphosphonates has shown its relevance, yet practical modalities still need to be defined. It would be interesting but quite difficult to implement, in light of the positive effect of this study, a prospective, randomized, controlled, double-blind vs. placebo study on a large enough sample of patients.
Résumé
L’ostéoporose est une complication classique chez les enfants déficients moteurs avec un risque élevé de fracture (20 % au cours de leur vie) essentiellement au niveau du fémur. Par ailleurs, les enfants présentent des douleurs sans que l’on ait pu faire le lien entre douleurs et ostéoporose. Le traitement par bisphosphonates a prouvé son efficacité chez l’adulte et dans l’ostéogenèse imparfaite de l’enfant. Son utilisation chez les enfants porteurs d’une déficience motrice reste à définir.
Méthodologie
Étude rétrospective sur dossiers d’enfants atteints de maladie neurologique entraînant une déficience motrice avec ostéoporose secondaire symptomatique ayant bénéficié d’un traitement par Pamidronate ® en intraveineux à Lyon et Valence entre 2002 et 2008. Recueil de l’effet sur les douleurs, la fréquence des fractures et la densité minérale osseuse. Recueil des effets secondaires du traitement pour évaluer sa tolérance.
Résultats
Douze enfants ont été étudiés avec au total 50 perfusions. Sur le plan des effets, nous observons une nette diminution voire une disparition des douleurs avec un effet bénéfique après 98 % des perfusions et une amélioration de la densité minérale osseuse (en lombaire, −46,5 % avant traitement et −27 % après traitement). Les effets secondaires restent limités et rapidement réversibles à type de syndrome pseudo grippal, douleurs musculaires et hypocalcémie asymptomatique.
Conclusion
Il apparaît essentiel de dépister les douleurs secondaires à l’ostéoporose chez ces enfants et de les traiter rapidement pour éviter l’impact sur la qualité de vie. Le traitement par bisphosphonates en intraveineux a montré son intérêt mais les modalités pratiques restent à définir. Il serait intéressant mais difficile à mettre en place, vu l’effet positif de cette étude, de réaliser une étude prospective randomisée, contrôlée, en double insu versus placébo avec un nombre de patients suffisant.
1
English version
1.1
Introduction
For a long time osteoporosis has been considered as an adult pathology. It is characterized by bone mass density (BMD) osteodensitometry measurements < −2.5 DS . To date, no specific pediatric definition for osteoporosis is available and the diagnosis in children is based on adult criteria. However, this pathology does affect children first because the life expectancy in these chronic pathologies has increased but also there is an increased use of long-term treatments inducing bone loss such as steroids .
All pathologies with motor impairments can induce osteoporosis in children. The risk is even higher if the child’s functional level is low . A study conducted in 2002 showed that 96% of children with GMF-CS 5 (maximal motor impairment in cerebral palsy) had BMD measurements < −2.5 DS at lumbar and femoral level as evidenced by osteodensitometry findings . The most frequent clinical pictures of osteoporosis in children with motor impairments are fractures and pain; consequently physiotherapy activities become more limited . Between 21 and 59% of these children will experience a fracture during their lifetime, often a femoral one, and these fractures are secondary to minimal trauma or no trauma at all . Their treatment rarely requires surgery, however they are a challenge for rehabilitation teams for these children and the subsequent immobilization aggravates this osteoporosis triggering recurrent fractures. In children, validated treatments for osteoporosis are based first on prevention by identifying risk factors and triggering circumstances for these fractures and secondly on calcium and vitamin D supplements to reach the target: 25OH vitamin D > 50 mmol/l .
In adults, bisphosphonates have proved their efficacy. This treatment has a validated impact on osteoporosis by inhibiting osteoclast activity and thus diminishing bone resorption . In children, studies have validated the effectiveness of bisphosphonates in treating osteoporosis for patients with osteogenesis imperfecta (OI) and reported as results: increased BMD from 0.5 to 1 DS per year that continued for 2 years after the end of the treatment, reduced number of fractures, decreased pain and increased mobility .
There are still very few data available regarding the effectiveness and tolerance of bisphosphonates in children with motor impairments, which motivated this study. The goal of this study on children with motor impairments and secondary osteoporosis was to describe the impact of I.V. bisphosphonates treatment on pain and number of fractures and evaluate treatment’s tolerance…
1.2
Patients and method
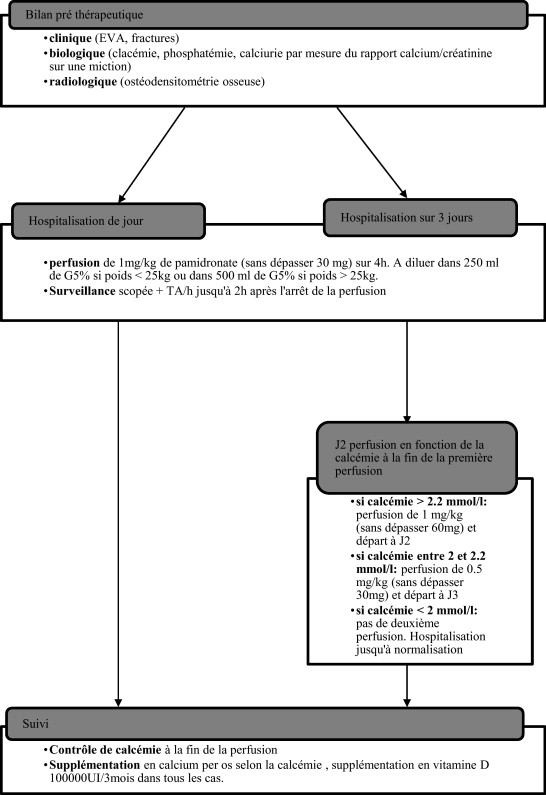
This retrospective study was based on data collected from patients’ medical charts. Inclusion criteria were: children with neuromuscular diseases and severe motor impairments leading to symptomatic secondary osteoporosis (facture and/or pain) who had treated by Pamidronate ® I.V. infusions in Lyon and Valence between 2002 and 2008 according to a standardized protocol in 1 or 3 consecutive days (2 days of I.V. infusion and 1 day of monitoring) ( Fig. 1 ). The treatment’s indications were: presence of debilitating pain, hard to control with the usual analgesic treatments. Clinical and biological monitoring was set up at the hospital (blood and urine tests for calcium and phosphate levels as well as urine calcium/creatinine ratios were done before and after treatment).
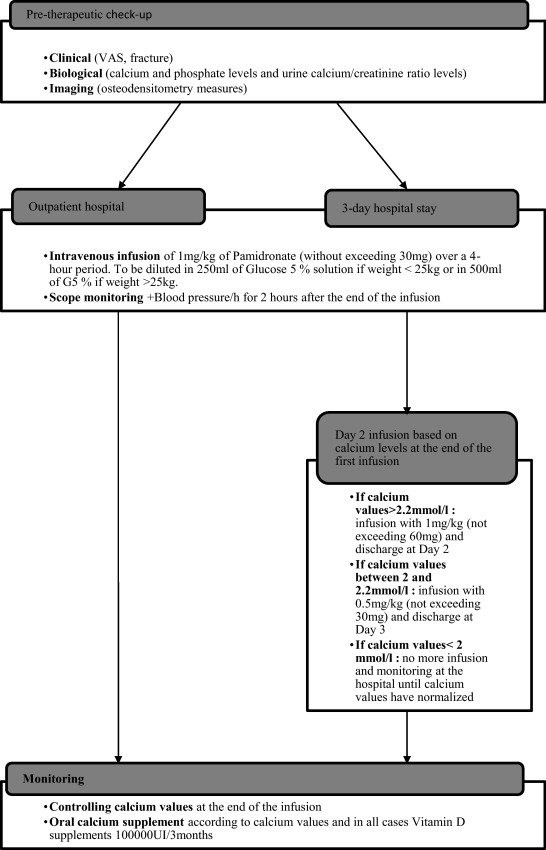
The choice of the I.V. infusion protocol was not established according to the severity of the child’s health status or original pathology but rather according to the care protocols of the hospital unit where the child was hospitalized. The treatment consisted in Pamidronate ® I.V. infusions with 1 mg/kg through I.V. over 1 to 2 consecutive days every three months, as recommended for the treatment of OI (i.e. 1 mg/kg per treatment and 4 mg/kg per year for the 1-day protocol or 2 mg/kg per treatment and 8 mg/kg per year for the 3-day protocol).
For each I.V. infusion, we collected data on pain (VAS pain scale or San Salvadour observational pain assessment scale before the beginning of the treatment than at Month 1 and Month 3 after treatment), the presence of fracture (before and after treatment) and BMD (measured with Dual-emission X-ray absorptiometry [DXA] of the spine). Adverse events of the treatment were also collected to evaluate its tolerance.
Because of the small number of patients in our study we reported qualitative data without mean averages. For quantitative data we used the Student’s t -test with an alpha risk at 0.05 to compare data before and after treatment.
1.3
Results
1.3.1
Population characteristics
Twelve children met the inclusion criteria: three girls and nine boys.
The patients’ characteristics are listed in Table 1 .
| Patient | Sex | Pathology | Motor status | Number of fractures before treatment | Age at the first fracture (years) | Duration of the pain before treatment (years) | Protocol type (day) | Total number of I.V. infusions | Age at the first I.V. (years) | Mean pain intensity before treatment | Mean pain intensity M1 after treatment | Mean pain intensity M3 after treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Glycogen storage disease typeII (Pompe disease) | Never walked | 4 | 2.8 | 1 | 1 | 4 | 8 | SSOPS 30 | SSOPS 8 | SSOPS 10 |
| 2 | M | DMD | Lost walking abilities at 10 | 0 | N/A | 1 | 3 | 2 | 14.5 | VAS 6 | VAS 0 | VAS 1 |
| 3 | M | DMD | Lost walking abilities at 9 | 1 | 3 | 0.5 | 3 | 1 | 13.5 | VAS 5 | VAS 5 | VAS 5 |
| 4 | F | Type 2 SMA | Never walked | 0 | N/A | 0.5 | 1 | 5 | 4.5 | VAS 8 | VAS 1 | VAS 2 |
| 5 | M | Type 2 SMA | Never walked | 3 | 4.7 | 4 | 3 | 4 | 9 | VAS 8 | VAS 0 | VAS 4 |
| 6 | M | DMD | Lost walking abilities at 7 | 0 | N/A | 3 | 1 | 21 | 13 | VAS 5 | VAS 0 | VAS 4 |
| 7 | F | Congenital MD | Never walked | 3 | 1.8 | 0.2 | 1 | 7 | 2.2 | SSOPS 22 | SSOPS 0 | SSOPS 3 |
| 8 | M | DMD | Lost walking abilities at 6 | 3 | 5.5 | 4 | 3 | 1 | 12.5 | VAS 6 | VAS 3 | VAS 4 |
| 9 | M | DMD | Lost walking abilities at 11 | 0 | N/A | 2 | 3 | 2 | 13 | VAS 9 | VAS 1 | VAS 3 |
| 10 | M | Type 2 SMA | Never walked | 1 | 11.5 | 4 | 3 | 1 | 12 | VAS 7 | VAS 3 | VAS 4 |
| 11 | M | DMD | Lost walking abilities at 11 | 0 | N/A | 0.5 | 3 | 1 | 13.6 | VAS 6 | VAS 0 | VAS 4 |
| 12 | M | DMD | Lost walking abilities at 11 | 0 | N/A | 0.5 | 3 | 1 | 14.3 | VAS 7 | VAS 4 | VAS 4 |
Pathologies responsible for the motor impairments of these children are: spinal muscular atrophy Type 2 (three), Duchenne muscular dystrophy (seven), glycogen storage disease type II (Pompe disease) (one), congenital muscular dystrophy (one).
All patients were non-walkers at the beginning of the treatment: five had never walked and seven had lost their walking abilities some years ago.
Half of these patients benefited from passive standing on regular basis, one hour per day in average, with a custom-molded assistive device.
All patients had physiotherapy two to three times a week, vitamin D (one blister every 3 months) and calcium supplements before the treatment was implemented.
1.3.2
Treatment indications
All children presented pain during joint mobilization, permanent pain or even severe pain disrupted their sleep and not managed by classic analgesics. Ten out of the 12 children in this study were taking analgesics regularly, the drugs used were acetaminophen (seven children were using it by itself), codeine (two children) and morphine (one child) yet this treatment could not provide enough pain relief which motivated the treatment with bisphosphonates (visual analog pain scale > 3 in spite of the analgesics used). The mean pain score before treatment for each patient is listed in Table 1 . The mean duration between the onset of pain and implementation of bisphosphonates treatment was 1.88 year (S.D.: 1.5 year).
Six out of the 12 children had had at least one fracture before the first infusion (one child had four fractures, three children had three fractures and two children had one). Amounting to 15 fractures observed for this cohort, 12 affecting the femur and three the humerus. The circumstances of onset for all these fractures involved very little or no impact (i.e., during transfers or physiotherapy sessions). The mean age at the first fracture was 4.88 years (S.D.: 3.51 years, range: 1.8–11.5 years).
1.3.3
Treatment modalities
These 12 children had a total of 50 Pamidronate ® I.V. infusions according to two different protocols, in 1 or 3 consecutive days. Eight children had the 3-day protocol and four children had the 1-day protocol. In case of validated efficacy and good tolerance, the treatment was repeated every 3 months. The total number of I.V. infusions varied (between 1 and 21 infusions) with a mean at 4.16 infusions per child i.e. one year of treatment in average. The mean age at the first I.V. infusion was 10.84 years (S.D.: 4.043 years and range: 2.2–14.5 years).
1.3.4
Lab tests before treatment
The pre-treatment blood test was normal: mean total calcium at 2.40 mmol/l (S.D.: 0.097) (normal range: 2.25 to 2.55 mmol/l), mean phosphate levels at 1.51 mmol/l (S.D.: 0.20) (NR: 1 to 1.8mmol/l), mean parathyroid hormone (PTH) levels at 32.23 pg/ml (S.D.: 15.05) (NR: 10 to 60 pg/ml), mean 25OH Vitamin D at 74.20 ng/ml (S.D.: 48.88) (NR: 15 to 40 ng/ml), mean alkaline phosphatase levels (APL) at 124.46 UI/l (S.D.: 21.39) (NR: 100 to 230 UI/l).
The only abnormality found was regarding urinary calcium/creatinine ratio values, with a mean at 1.44 mmol/mmol (S.D.: 0.83) i.e. far above the normal range for children over the age of 4 which is set at < 0.3 mmol/mmol.
1.3.5
Treatment effects
1.3.5.1
On pain control
In 20% of cases (10/50), there was complete pain relief after treatment (VAS at 0 at Month 1 and Month 3 after treatment), in 60% of the cases (30/50), there was transitory pain relief (EVA at 0 at M1 and > 3 at M3 after treatment) and in 18% of cases, the pain had decreased by more than three points on the VAS at M1 and M3. In only 2% of the cases (1/50), the infusions were ineffective ( Fig. 2 ). The global result on pain was effectiveness in 98% of the cases ( Fig. 3 ).
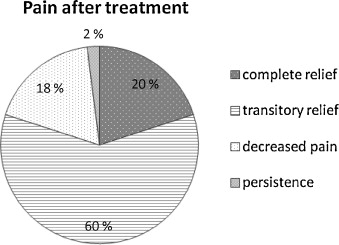
When the treatment brought an improvement, it was an immediate one. The mean pain scores for each patient at M1 and M3 are listed in Table 1 .
1.3.5.2
On fractures
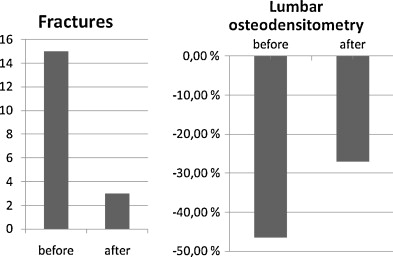
Six children out of 12 presented at least one fracture before the treatment (with a mean follow-up up between 1 and 10 years). After the treatment, 10 out of 12 children had no more fractures. Two children had additional factures after treatment most often affecting the femur ( Fig. 4 ); the first child presented two fractures immediately after the first infusion, the second 6 months after a well-conducted treatment. We were able to collect a mean follow-up of 1.7 year.
1.3.5.3
On BMD
For four children, we were able to collect osteodensitometry data before and after treatment. For these four children, we observed a clear BMD improvement at lumbar level which went from −46.5 to −27% of normal BDM according to age and weight ( p = 0.05) ( Fig. 4 ). Given the retrospective nature of the study, we did not have any other osteodensitometry data for the other eight patients studied.
1.3.6
Treatment’s tolerance
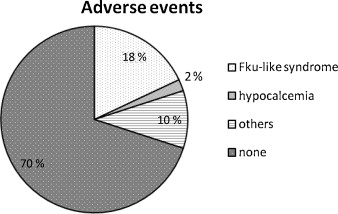
Essentially, the main adverse event reported was a flu-like syndrome during the first I.V. infusion or up to 48 hours after the beginning of the treatment. Nine infusions out of 50 triggered this type of adverse event that spontaneously resolved in a few hours. Only one infusion triggered hypocalcemia and was stopped. The other adverse side events reported were muscle pain (three infusions out of 50), headaches (1/50) and vomiting (1/50): all of which were resolved in less than 48 hours after the end of the infusion. No incidence of jaw osteonecrosis was reported.
Regarding biological results, we observed a significant decrease of the calcium levels after treatment (mean at 2.4 mmol/l before treatment and 2.22 mmol/l after treatment) ( t = 6.337, p < 0.0001); as well as a significant decrease of phosphate levels (mean at 1.51 mmol/l before treatment and 1.32 mmol/l after treatment) ( t = 4.340, p < 0.0001): yet never reaching a critical threshold requiring specific care ( Fig. 5 ).
1.4
Discussion
Our study is one of the first to study the effectiveness of this treatment on pain and the positive results of the treatment speak for themselves with 98% of infusions leading to improvement or even total alleviation of the pain when these children had been suffering for several months with no relief from classic analgesics. In fact, not only did this treatment improve their quality of life, but it permitted better and more effective rehabilitation training sessions (the latter were often neglected due to the pain).
Previous studies published mostly focused on the effectiveness on BMD measured by osteodensitometry, the only objective quantitative criterion used mostly in adults . However, BMD is not a good criterion in children with motor impairments because it is not correlated to the risk of fracture . Furthermore, osteodensitometry measurements are often done at lumbar level when osteoporosis in children with motor impairments is mostly observed on the femoral neck. It has been validated that lumbar BMD measures cannot be extrapolated to the entire body . In our study, the treatment was effective on lumbar BMD, but due to the low number of patients we cannot reach any significant conclusion.
The decreased number of fractures after the treatment seems quite real. Yet our retrospective study only had a 6-year follow-up and thus yielded an insufficient level of evidence to validate this result, just like the other studies conducted to date . We should underline that not all our patients benefited from the regular Pamidronate ® I.V. treatment every 3 months for 1 to 2 years. This could also explain the high rate of fracture recurrence in spite of the treatment.
Treatment tolerance was rather good since the most frequent adverse event was a well-tolerated flu-like episode that spontaneously resolved over a few hours. This result is in accordance with the previously published studies. Hypocalcemia events were rare and asymptomatic . However, in pediatric care, no evidence of jaw osteonecrosis was reported after bisphosphonates treatment . A review of the literature published in reported the risk of jaw osteonecrosis after bisphosphonates treatment at 1/100000 per year in adults. However, no relation was ever established with jaw osteonecrosis and bisphosphonates in osteoporosis care management. For Rizzoli et al., in light of these data no specific dental care should be implemented before treatment .
One study showed a lower treatment tolerance in patients with Duchenne muscular dystrophy, which was not evidenced in our study .
On a biological level, the higher urinary calcium/creatinine rate (Ca/Cr) values before treatment would seem to be the consequence of immobilization . The main weakness of this study is its small sample size. This small cohort prevented us from comparing the effectiveness of these two protocols (1 or 3 days), as studied in a previous publication . This study conducted for children with OI showed an increase in BMD and a decreased risk of fracture after the 1-day treatment. This still remains to be defined but could improve patients’ tolerance and compliance to the treatment.
Finally, it would be relevant to conduct prospective studies to compare the efficacy and tolerance of the various bisphosphonates’ molecules available, doses to be administered and administration modes. In fact for a similar effectiveness, an oral administration of the treatment at home would seem preferable than I.V. protocols in a hospital setting.
1.5
Conclusion
It seems quite essential to detect pain in children with motor impairments and implement a treatment very quickly to avoid a negative impact on their quality of life. Treatment with bisphosphonates I.V. infusions has shown its relevance yet some additional studies are necessary to evaluate the costs and constraints related to hospital stay and the effectiveness of the treatment on pain according to the patients and their family. This assessment could help define the best therapeutic protocol. Finally, it would be quite relevant to compare this effectiveness with an oral administration of the treatment.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
2
Version française
2.1
Introduction
L’ostéoporose, longtemps considérée comme une pathologie de l’adulte, est caractérisée par une densité minérale osseuse (DMO) inférieure à −2,5 DS sur l’ostéodensitométrie osseuse . Il n’existe pas de définition spécifiquement pédiatrique et actuellement le diagnostic est posé chez l’enfant en se basant sur les critères adultes. Pourtant cette pathologie touche aujourd’hui aussi l’enfant en raison de l’augmentation de l’espérance de vie dans certaines pathologies chroniques et de l’utilisation au long cours de traitements ostéopéniant comme les corticoïdes . Toutes les pathologies responsables d’une déficience motrice sont à risque d’ostéoporose chez l’enfant. Le risque est d’autant plus important que le niveau fonctionnel est bas . Une étude réalisée en 2002 montre que 96 % des enfants de niveau GMF-CS 5 (handicap moteur maximal dans la paralysie cérébrale) ont une DMO inférieure à −2,5 DS sur l’ostéodensitométrie osseuse en lombaire et en fémoral . Les manifestations cliniques les plus fréquentes de l’ostéoporose chez l’enfant déficient moteur sont les fractures, les douleurs ; on observe, de plus, une limitation des interventions de kinésithérapie secondaires à celles-ci . Entre 21 et 59 pour cent de ces enfants ont une fracture au cours de leur vie avec une localisation préférentielle au fémur et ces fractures sont secondaires à des traumatismes minimes ou inexistants . Leur traitement est rarement chirurgical mais elles constituent un problème majeur dans les centres de rééducation prenant en charge ces enfants et l’immobilisation qui en découle aggrave encore l’ostéoporose entraînant parfois des fractures à répétition. Les traitements reconnus pour l’ostéoporose de l’enfant sont en premier lieu le traitement des facteurs de risque et de la cause, la supplémentation en calcium et en vitamine D avec comme objectif un dosage sanguin de 25OH vitamine D > 50 mmol/L . Chez l’adulte, les bisphosphonates ont également fait la preuve de leur efficacité. Ce traitement a un effet reconnu en inhibant les ostéoclastes et diminuant, de ce fait, la résorption osseuse . Chez l’enfant, des études ont montré l’efficacité des bisphosphonates dans le traitement de l’ostéoporose chez les patients porteurs d’ostéogenèse imparfaite avec une augmentation de la densité osseuse de 0,5 à 1 DS par an qui persiste même deux ans après la fin du traitement, une diminution du nombre de fractures, une diminution des douleurs et une augmentation de la mobilité .
Les données sont encore rares concernant l’efficacité et la tolérance du traitement par bisphosphonates chez les enfants porteurs de déficience motrice, ce qui justifie cette étude. L’objectif de cette étude est de décrire, dans une population d’enfants porteurs d’une déficience motrice avec ostéoporose, l’effet d’un traitement intraveineux par bisphosphonates sur la douleur, le nombre de fracture et sa tolérance.
2.2
Patients et méthode
Il s’agit d’une étude rétrospective sur dossiers. Ont été inclus les enfants atteints de maladie neuromusculaire entraînant une déficience motrice sévère avec ostéoporose secondaire symptomatique (fracture et/ou douleur) ayant bénéficié d’un traitement par Pamidronate ® en intraveineux à Lyon et à Valence entre 2002 et 2008 selon un protocole précis en un ou trois jours consécutifs (deux jours de perfusions et un de surveillance) ( Fig. 1 ). Les indications du traitement reposaient sur la présence de douleurs invalidantes difficiles à gérer avec les traitements antalgiques habituels. Une surveillance clinique et biologique (calcémie, phosphatémie, calciurie avant et après traitement) est mise en place durant l’hospitalisation.