Abstract
Objectives
Complex regional pain syndrome type 1 (CRPS-1) can progress to joint stiffness, which may be related to pain and/or capsule-ligament contracture. In this context, it is difficult to distinguish the respective causative roles of pain and contractures. Nerve blocks (NBs) can be used to determine the aetiology of joint stiffness. Subsequent treatment will depend on whether contractures are present or not. The objective of the present study was to evaluate the diagnostic and therapeutic value of the nerve blocks in the management of joint stiffness caused by CRPS-1.
Design of the study
A retrospective case series.
Methods
Implementation of NBs in subjects with joint stiffness caused by CRPS-1. Primary efficacy criterion: an increase in the range of joint movement. Secondary criteria: pain level, treatment decision, duration of therapeutic NBs, return to work.
Results
Fourteen patients with joint stiffness underwent 17 NBs. Ten NBs (59%) were associated with the normalization of the range of joint movement (i.e. the absence of contractures and the presence of an isolated pain component), prompting the implementation of physical therapy during NBs (“therapeutic NBs”) in 90% of these cases. Three NBs (18%) were associated with a partial increase in the range of joint movement (i.e. a background of joint stiffness due to a combination of pain and contracture), prompting the implementation of a therapeutic NB in all of these cases. Four NBs (23%) were not associated with any increase in the range of joint movement (i.e. pure contractures), prompting consultation with a surgeon in all of these cases. Forty-three percent of the patients have since returned to work.
Conclusions
Nerve block is a valuable diagnostic and therapeutic option in the management of joint stiffness caused by CRPS-1.
Résumé
Objectifs
Le syndrome douloureux régional complexe de type I (SDRC I) est responsable de douleurs et peut évoluer vers une raideur articulaire. Cette raideur peut être d’origine algique ou liée à des rétractions capsulo-ligamentaires. Il est difficile de faire la part entre douleurs et rétractions. Les blocs nerveux périphériques (BNP) peuvent préciser l’étiologie de la raideur. Selon l’existence ou non de rétractions, la prise en charge sera différente. Notre objectif est d’évaluer l’intérêt diagnostique et thérapeutique des BNP dans la prise en charge de raideurs articulaires liées au SDRC I.
Design de l’étude
Étude rétrospective, série de cas.
Méthodes
Réalisation de BNP chez des sujets présentant une raideur articulaire liée à un SDRC I. Critère de jugement principal : progression des amplitudes articulaires. Critères secondaires : douleur, décision thérapeutique, durée du BNP, reprise professionnelle.
Résultats
Quatorze patients avec raideur articulaire ont bénéficié de 17 BNP : 10 BNP (59 %) ont montré une normalisation des amplitudes articulaires (absence de rétraction, composante douloureuse pure), aboutissant pour 90 % d’entre eux à une kinésithérapie sous bloc (BNP thérapeutique). Trois BNP (18 %) ont montré une amélioration partielle des amplitudes articulaires (association douleur + rétractions), conduisant tous à un BNP thérapeutique. Quatre BNP (23 %) ont montré une absence d’amélioration des amplitudes articulaires (rétractions pures), aboutissant tous à un avis chirurgical. Quarante-trois pour cent des patients ont repris une activité professionnelle.
Conclusions
Les BNP représentent une alternative diagnostique et thérapeutique dans la prise en charge de raideurs articulaires liées au SDRC I.
1
English version
1.1
Introduction
Complex regional pain syndrome type I (CRPS-1) is associated with pain and vasomotor and trophic phenomena. In the mid-term, joint stiffness can appear; this may be caused by pain alone or by true capsule and ligament contractures and/or muscle and tendon contractures. It is sometimes difficult to distinguish between the respective causative roles of pain and contractures. The treatment of CRPS-1 is often challenging, with a large number of poorly codified therapeutic approaches. In the literature, only oral and intravenous bisphosphonates have proven efficacy . The levels of evidence for calcitonin, corticosteroids, gabapentin, vasodilators, sympatholytic drugs and stellate or lumbar sympathetic ganglion blocks are not high enough for their recommendation in the treatment of CRPS-1. The same is true for physical therapy and occupational therapy .
The use of nerve blocks (NBs) in the management of joint stiffness due to CRPS-1 has not been extensively documented. However, NBs may have value in both diagnosis (by distinguishing between the respective roles of pain and capsule/ligament contracture in joint stiffness) and therapy (by providing pain relief and thus enabling physical therapy during the block). Literature data on this subject are scare and relate only to case reports .
The objective of the present study was thus to assess the diagnostic and therapeutic value of NBs in patients presenting joint stiffness in a context of CRPS-1.
1.2
Methods
This was a descriptive, retrospective case series in patients presenting joint stiffness caused by CRPS-1 and having undergone NBs in the Physical and Rehabilitation Medicine (PRM) Department at Strasbourg University Hospital (Strasbourg, France) between January 2004 and January 2011.
1.2.1
Characteristics of the study population
We collected:
- •
clinical, sociodemographic and psychological data on the population;
- •
data related to CRPS-1 and joint stiffness;
- •
data on the NB itself.
1.2.1.1
Clinical data
All the patients had a diagnosis of CRPS-1 with stiffness affecting one or more limb joints.
The diagnosis of CRPS-1 was based on clinical criteria (disease history, neuropathic pain, vasomotor and/or trophic disorders, etc.) and (in some cases) confirmation by bone scintigraphy.
Joint stiffness was measured with conventional goniometry or by noting abnormal postures.
The exclusion criteria were as follows:
- •
allergy to the local anaesthetics lidocaine or ropivacaine;
- •
spontaneous or induced coagulation disorders;
- •
any concomitant, progressive systemic disorder;
- •
skin infection at the puncture point;
- •
lack of cooperation.
After receiving comprehensive information on the study’s objectives and procedures, the patients gave their written, informed consent to participation.
1.2.1.2
Sociodemographic and psychological data
The following types of sociodemographic data were collected: the number of patients, gender, age, and socioprofessional context (i.e. occurrence of a workplace accident or an occupational disease).
Psychological data were taken from a psychiatric consultation arranged for the patient when the PRM physician suspected the presence of psychological disorders.
1.2.1.3
Data related to CRPS-1 and the joint stiffness
The data related to CRPS-1 and joint stiffness were as follows:
- •
the aetiology of CRPS-1;
- •
the time since onset of CRPS-1;
- •
the site(s) of the joint stiffness related to CRPS-1;
- •
treatment(s) prior to the NB.
1.2.1.4
Data related to the NB
We recorded the topography of each NB (i.e. the anaesthetized nerves and the approach used).
1.2.2
Procedure
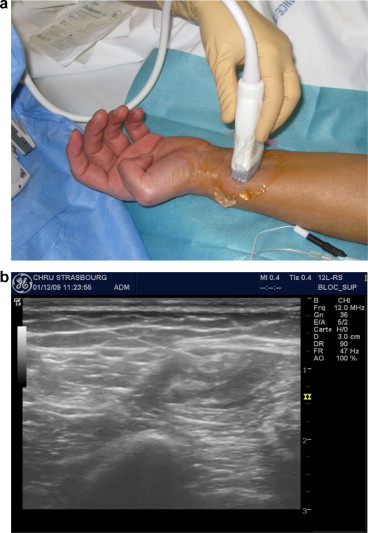
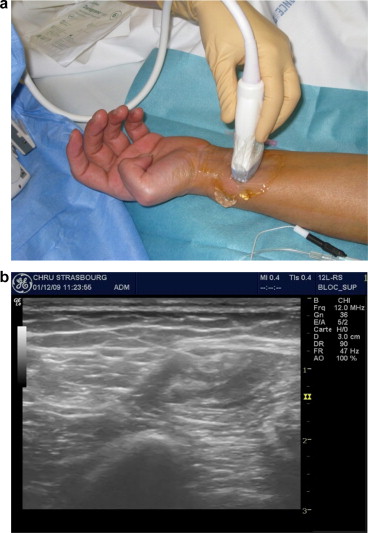
The NB was performed under optimal safety conditions at Strasbourg University Hospital by an anesthetist with expertise in local/regional anesthesia. The procedure took place in a surgical recovery room, with intensive care facilities nearby. The patient was monitored for vital signs (electrocardiogram, blood pressure, and blood oxygen saturation) and peripheral venous assess was implemented. The nerve to be anaesthetised was identified ( Fig. 1 a and b) using skin surface landmarks (drawn on the skin with a dermographic pen). Neurostimulation was performed with a Stimuplex ® HSN12 system (B. Braun Melsungen AG, Melsungen Germany; minimum stimulation intensity: 0.4 mA), suitable needles (Stimuplex ® (B Braun Melsungen AG) for diagnostic NB and Contiplex ® (B Braun Melsungen AG) for therapeutic NBs) and ultrasound guidance [with a LOGIQ e-ultrasound system (GE Healthcare, Chalfont St Giles, UK)]. The anesthetist identified the nerve and the surrounding structures and monitored the progression of the needle and the diffusion of the local anesthetic in real time.
The NB’s effectiveness was always checked with an ice cube test (i.e. cold insensitivity when the NB was effective).
For a diagnostic NB, the local anesthetic used was 1% lidocaine (Xylocaine ® ) administered perineurally through a catheter.
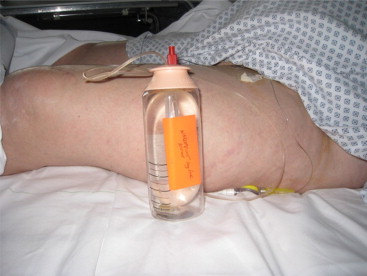
The PRM physician came to the recovery room 30 to 60 minutes after the implementation of the diagnostic NB and recorded any changes in the range of joint movement. In the event of a total or partial normalization of the range of joint movement, the PRM physician concluded that the joint stiffness had been fully or partially due to pain and thus suggested the implementation of a long-term therapeutic NB (to enable rehabilitation during analgesia). The local anesthetic used was 0.75% ropivacaine (Naropeine ® ), delivered continuously by an elastomeric pump (flow rate: 5 mL per hour) through a perineural tunnel catheter ( Fig. 2 ). In such a case (and two hours of monitoring in the recovery room), the patient was hospitalized in the PRM Department and remained there as long as the NB was effective. The patient then underwent two one-hour physical therapy sessions a day, with mobilisation of the limb segments affected by joint stiffness. Furthermore, the patient was asked to flex and extend the affected joints him/herself as much as possible between the physical therapy sessions. To ensure that the catheter had not moved, the care team supervised the patient’s transfers and movements closely. A tunnel catheter was used to reduce the risk of subsequent movement. The care team checked the entry point daily for local inflammation. An anesthetist was available round the clock in the event of NB-related problems.
1.2.3
Evaluation and follow-up
1.2.3.1
Primary efficacy criterion: an increase in the range of joint movement
The primary efficacy criterion was the increase in the range of joint movement after NB, with three possible ratings:
- •
normalization of the range of joint movement after NB: recovery of a normal range of joint movement (testifying an isolated pain component in the limb or limb segment stiffness, and thus the total absence of contractures);
- •
a partial increase in the range of joint movement: a partial decrease in joint stiffness but not enough to achieve a normal range of joint movement (testifying a combined role of pain and capsule/ligament contracture in the joint stiffness);
- •
no increase in the range of joint movement: no change in joint stiffness (testifying isolated capsule/ligament contractures).
1.2.3.2
Secondary evaluation criteria
The secondary evaluation criteria were as follows:
- •
the pain level after the NB, evaluated as a binary parameter (i.e. a decrease in pain or no change in pain);
- •
the treatment decision after the diagnostic NB (therapeutic NB or consultation with a surgeon);
- •
the duration of therapeutic NB, if implemented;
- •
the return to work (evaluated via a telephone interview 6 months later).
1.2.4
Data analysis
Depending on the parameter, the descriptive characteristics of the study population are reported as a percentage or as the mean ± standard deviation (SD) [range]. Descriptive analyses were performed with Sigma Stat software (version 3.5, Systat Software Inc., San Jose, CA, USA).
1.3
Results
1.3.1
Characteristics of the study population
The 14 patients included in our study underwent a total of 17 NBs between January 2004 and January 2011. Twelve patients underwent one NB, one patient underwent two NBs and one patient underwent three NBs.
1.3.1.1
Clinical data
The 14 included subjects all presented a clinical diagnosis of CRPS-1, with a compatible history of disease (trauma or initial surgery), neuropathic pain, and vasomotor or trophic disorders. In 8 of the 14 patients, the diagnosis of CRPS-1 had been confirmed by bone scintigraphy.
Furthermore, we noted joint stiffness (the inclusion criterion) in all 14 patients but were unable to clinically determine the respective causative roles of pain and fixed contractures.
1.3.1.2
Sociodemographic and psychological data
The patients’ sociodemographic data (age, gender, professional context as a workplace accident or occupational disease) are summarized in Table 1 .
| Age (years) | 45 ± 10 [32–80] |
| Gender (male/female) | 6/8 (42.9%–57.1%) |
| Professional context: workplace accident or occupational disease | 9 (64.3%) |
In terms of the patients’ psychological status, eight had consulted a psychiatrist following a recommendation by the PRM physician. The psychiatrist diagnosed a psychiatric condition in seven patients: hysterical neurosis ( n = 4), depressive syndrome ( n = 2), and malingering for secondary gain ( n = 1). The eight patients who did not consult a psychiatrist did not display any psychological disorders (according to the PRM physician).
1.3.1.3
Data related to CRPS-1 and joint stiffness
The aetiology of CRPS-1, its time since onset and the site of joint stiffness are summarized in Table 2 . The mean ± SD time since onset of CRPS-1 was 2.75 ± 2.2 years (0.5–8). The treatments prior to the implementation of NB are listed in Table 3 .
| Aetiology of CRPS-1 | Time since onset of CRPS-1 (years) | Site of joint stiffness | |
|---|---|---|---|
| Patient 1 | Partial meniscectomy | 3 | Knee flexion deformity (45°) |
| Patient 2 | Mediotarsal luxation fracture, treated orthopedically | 1 | Ankle equinus deformity (35°) |
| Patient 3 | Ankle fracture, treated orthopedically | 2 | Ankle equinus deformity (30°) |
| Patient 4 | Neurolysis of the median nerve to the carpal tunnel | 1 | Frozen shoulder, impaired supination, wrist flexion deformity, dual flexion deformities of all five fingers (MCP and PIP) |
| Patient 5 | Partial meniscectomy | 5 | Knee flexion deformity (90°) |
| Patient 6 | Fracture at the distal end of the radius, treated orthopedically | 1 | Frozen shoulder, impaired pronation, wrist flexion deformity (30°), dual flexion deformities of all five fingers (MCP and PIP) |
| Patient 7 | Fracture of the left calcaneus, treated with osteosynthesis | 3 | Ankle equinus deformity (35°) |
| Patient 8 | Deep cut to the thumb, treated with surgery | 3 | Dual flexion deformities of the thumb (MCP + IP) |
| Patient 9 | Rheumatoid synovitis of the knee | 1 | Knee flexion deformity (30°) |
| Patient 10 | Rupture of the anterior cruciate ligament, treated with ligamentoplasty of the fascia lata | 0.5 | Knee flexion deformity (20°) |
| Patient 11 | Shoulder joint replacement prompted by arthrosis | 2 | Frozen shoulder, elbow flexion deformity (90°), dual flexion deformities of all five fingers (90°) (MCP + PIP) |
| Patient 12 | Rupture of the glenoidal labrum, sutured surgically | 6 | Frozen shoulder |
| Patient 13 | Severe ankle sprain, treated orthopedically | 8 | Foot and toe stiffness |
| Patient 14 | Incomplete asymmetric tetraplegia after C6 fracture, not operated | 2 | Flexion deformities of the PIP joints of all five fingers |
| Step 1 analgesia | Step 2 analgesia | Step 3 analgesia | SC calcitonin | Oral NSAIDs | Corticoid infusion | Anti-epileptics | IV BP | Physic. ther | |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | X | X | X | X | X | X | |||
| Patient 2 | X | X | X | X | X | ||||
| Patient 3 | X | X | X | X | X | ||||
| Patient 4 | X | X | X | X | X | X | |||
| Patient 5 | X | X | X | X | |||||
| Patient 6 | X | X | X | ||||||
| Patient 7 | X | X | X | ||||||
| Patient 8 | X | X | X | ||||||
| Patient 9 | X | X | X | X | |||||
| Pat. 10 | X | X | X | X | X | ||||
| Pat. 11 | X | X | X | ||||||
| Pat. 12 | X | X | X | X | X | X | |||
| Pat. 13 | X | X | X | ||||||
| Pat. 14 | X | X | X |
1.3.1.4
Data in rapport with the NB
The 17 NBs were initially performed for diagnostic purposes, in order to determine the respective causative roles of pain and contractures in the joint stiffness. When the outcome of this diagnostic NB revealed the presence of a pain component (even when partial), a long-term therapeutic NB was suggested to the patient in order to enable rehabilitation during analgesia.
Table 4 details the topographies and outcomes of the 17 NBs.
| Site of joint stiffness | Topography of the NB | Change in the range of joint movement | Treatment decision (duration of therapeutic NB) | |
|---|---|---|---|---|
| Patient 1 | Knee flexion deformity (45°) | Femoral nerve block + sciatic nerve block with a parasacral approach | Normalization of the range of knee joint movement | Therapeutic NB (7 days) |
| Patient 2 NB 1 | Ankle equinus deformity (35°) | Tibial nerve block in the popliteal fossa | Normalization of the range of ankle joint movement | Therapeutic NB (7 days) |
| Patient 2 NB 2 | Ankle equinus deformity (35°) | Tibial nerve block in the popliteal fossa | Normalization of the range of ankle joint movement | Therapeutic NB (7 days) |
| Patient 3 | Ankle equinus deformity (30°) | Tibial nerve block in the popliteal fossa | Normalization of the range of ankle joint movement | Therapeutic NB (3 days) |
| Patient 4 | Frozen shoulder, impaired supination, wrist flexion deformity, dual flexion deformities of all five fingers (MCP and PIP) | Brachial plexus block with an infraclavicular approach | Absence of increase in the range of arm joint movement | Consultation with a surgeon: no surgical treatment |
| Patient 5 NB 1 | Knee flexion deformity (90°) | Femoral nerve block + sciatic nerve block with a parasacral approach | Normalization of the range of knee joint movement | Therapeutic NB (6 days) |
| Patient 5 NB 2 | Knee flexion deformity (90°) | Lumbar plexus block with a paralumbar approach + sciatic nerve block with a parasacral approach | Normalization of the range of knee joint movement | Therapeutic NB (7 days) |
| Patient 5 NB 3 | Knee flexion deformity (90°) | Lumbar plexus block with a paralumbar approach + sciatic nerve block with a parasacral approach | Partial increase in the range of knee joint movement (incomplete reduction of the knee flexion deformity) | Therapeutic NB (3 days) |
| Patient 6 | Frozen shoulder, impaired pronation, wrist flexion deformity (30°), dual flexion deformities of all five fingers (MCP and PIP) | Brachial plexus block with an intraclavicular approach | Partial increase in the range of joint movement: increase in pronation, complete reduction of the wrist flexion deformity and complete reduction of the flexion deformity of the MCP and PIP joints of the 4th and 5th fingers | Therapeutic NB (5 days) |
| Patient 7 | Ankle equinus deformity (35°) | Tibial nerve block to the popliteal fossa | Absence of an increase in the range of ankle joint movement | Consultation with a surgeon: no surgical treatment |
| Patient 8 | Dual flexion deformities of the thumb (MCP + PIP) | Median nerve block at the wrist | Absence of an increase in the range of thumb joint movement | Consultation with a surgeon: tenotomy |
| Patient 9 | Knee flexion deformity (30°) | Lumbar plexus block with a paralumbar approach + sciatic nerve block with a parasacral approach | Partial increase in the range of knee joint movement | Therapeutic NB (17 days) |
| Patient 10 | Knee flexion deformity (20°) | Lumbar plexus block with a paralumbar approach + sciatic nerve block with a parasacral approach | Normalization of the range of knee joint movement | Therapeutic NB (4 days) |
| Patient 11 | Frozen shoulder, elbow flexion deformity (90°), dual flexion deformities of all five fingers (MCP + PIP) | Brachial plexus block with a supraclavicular approach + medio-ulnar block | Normalization of the range of arm joint movement | Refusal of therapeutic NB |
| Patient 12 | Frozen shoulder | Brachial plexus block with an intraclavicular approach | Normalization of the range of shoulder joint movement | Therapeutic NB (not tolerated after day 1, so withdrawn) |
| Patient 13 | Foot and toe stiffness | Tibial nerve block | Normalization of the range of joint movement | Therapeutic NB (6 days) |
| Patient 14 | Flexion deformities of the PIP joints of all five fingers | Medio-ulnar block | Absence of increase in the range of joint movement | Consultation with a surgeon regarding tenotomy |
It is important to note that there were no complications following the implementation of these NBs.
1.3.2
Outcome of the NB
The main outcomes of the NB are summarized in Table 4 .
1.3.2.1
Primary evaluation criterion: an increase in the range of joint movement
Ten NBs (59%) were associated with a normalization of the range of joint movement (corresponding to an isolated pain component and no contractures).
Three NBs (18%) were associated with a partial increase in the range of joint movement (without achieving normative values and corresponding to a mixture of pain- and contracture-related components).
Four NBs (23%) were not associated with any increase in the range of joint movement (corresponding to joint stiffness caused by fixed contractures).
1.3.2.2
Secondary evaluation criteria
1.3.2.2.1
Treatment decision
Nine of the 10 NBs associated with a normalization of the range of joint movement (i.e. the absence of contractures) prompted the implementation of a long-term therapeutic NB and concomitant rehabilitation. One patient refused a therapeutic NB because he did not wish to stay in hospital.
All three NBs associated with a partial increase in the range of joint movement (i.e. some degree of contracture) prompted the implementation of a long-term therapeutic NB and concomitant rehabilitation.
All four NBs, not associated with any increase in the range of joint movement (i.e. the existence of fixed contractures), prompted the withdrawal of the block and consultation with a surgeon. This led to two tenotomies (tendon lengthening), whereas treatment was withheld in the two other instances because of associated comorbidities.
Overall, the 17 diagnostic NBs prompted a recommendation of therapeutic NB in 13 patients (one of whom refused) and thus resulted in the implementation of 12 therapeutic NBs with rehabilitation.
1.3.2.2.2
Pain after NBs
In 12 instances (71%), NBs was associated with a decrease in pain levels. In 5 NBs (29%), the pain level did not change.
1.3.2.2.3
The duration of therapeutic NBs
The mean duration of therapeutic NB was 6 ± 4 days ( Table 4 ). It is noteworthy that one therapeutic NB was curtailed after one day because the patient (patient 12) could not stand the sight of the catheter.
1.3.2.2.4
Return to work
Six patients (43%) returned to work [4 full-time (29%) and 2 part-time (14%)]. Of the 8 remaining patients, 2 were retired, 4 went onto invalidity benefits and 2 had missing data in this respect.
1.4
Discussion
Our study results show that NBs has both diagnostic and therapeutic value in the management of joint stiffness in patients with CRPS-1.
In this indication (and from a diagnostic point of view), NBs can help the physician to distinguish between pain and capsule/ligament contractures. In turn, this helps to guide the treatment decision, with either physical therapy (active and passive mobilization during long-term NBs) in cases with pain or surgical treatment in cases with fixed capsule/ligament contractures (tenotomy/tendon lengthening). Even though this diagnostic approach is known and has been used to treat neuro-orthopaedic disorders related to spasticity , we did not find any literature data on the diagnostic value of NBs in joint stiffness caused by CRPS-1. Our present study also emphasized therapeutic role of NBs, with the implementation of 12 long-term NBs that enabled physical therapy during the resulting analgesia. Pain levels fell in two-thirds of the cases. The mean duration of continuous NB was 6 days; all the patients then continued physical therapy on an outpatient basis for at least 3 months. Six patients were able to return to work.
Although our outcomes were mixed, they were similar to those reported by other researchers and may give hope to patients in whom other treatments have failed. James et al. assessed the value of NBs of the brachial plexus and physical therapy in 25 patients suffering from chronic upper extremity pain. The researchers noted a decrease in pain levels and an increase in the range of joint movement in 17 patients. Ribbers et al. described a partial decrease in pain and improved function in six women suffering from CRPS-1 in the arm after NBs of the brachial plexus. Dadure et al. evaluated the value of NBs (combined with sympathetic blocks and physical therapy) in 13 children suffering from CRPS-1 in the limbs. Two months later, the children and their parents reported a high degree of treatment satisfaction (with complete disappearance of pain and the recovery of normal function). Detaille et al. reported on a study on 59 subjects suffering from CRPS-1 in the arm; treatment with NBs of the brachial plexus and physical therapy was associated with a decrease in pain, improved function and a greater range of joint movement in the short- and medium-term in 86% of the patients. Forty-six percent of the patients returned to work. Lastly, Toshniwal et al. studied 33 patients suffering from CRPS-1 of the upper limbs and who had been treated by continuous stellate or lumbar sympathetic ganglion block or continuous infraclavicular brachial plexus block with bupivacaine for a week. Both groups experienced a significant reduction in neuropathic pain and oedema and a greater range of joint movement. Toshniwal et al.’s results suggest that NB is not inferior to sympathetic ganglion blocks, which were previously considered to be one of the reference treatments for CRPS-1 and had been extensively described in the literature.
Our results may have been affected by several sources of bias, due to the study’s retrospective design and the small sample size. Nevertheless, it is similar to other studies in this field (notably in terms of the sample size). The dual diagnostic and therapeutic role of NBs justifies the use of this technique in patients with CRPS-1 and who are unable to undergo treatment because of pain and abnormal posture.
Moreover, it must be borne in mind that a patient’s socioprofessional context and psychological status have a significant impact on the persistence of pain. In the present study, over half of the patients had suffered from a workplace accident or an occupational disease. Furthermore, 50% presented psychological disorders (with a background of anxiety/depression or somatoform disorders). This observation agrees with the meta-analysis by Beerthuizen et al. , which concluded that subjects having experienced more “life events” had a greater chance of developing CRPS-1.
The non-negligible role of the socioprofessional context and psychological status may explain the persistence of pain after NBs in one third of the cases and the low proportion of patients who returned to work (43%). This may be related to the multifactorial dimension of pain.
Lastly, our technique for implementing NB with ultrasound guidance complies with the current guidelines, with ultrasound being described as the “gold standard” in the literature .
These initial encouraging results must be confirmed in further research. In the future, it may be appropriate to compare actively treated patients with a control group. Bogduk et al. looked at this issue and stated that any anesthetic block should be compared with injections of physiological saline solution or of another local anesthetic, in order to avoid false positives caused by a placebo effect.
Subject to the results of further research, combined diagnostic and therapeutic NB may be a valuable treatment option for patients suffering from joint stiffness caused by CRPS-1.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
2
Version française
2.1
Introduction
Le syndrome douloureux régional complexe de type I (SDRC I) est une affection responsable de phénomènes douloureux, vasomoteurs et trophiques. À moyen terme peuvent survenir des raideurs articulaires, liées soit aux douleurs, soit à de réelles rétractions capsulo-ligamentaires et/ou musculo-tendineuses. Il est quelquefois difficile de faire la part entre douleurs et rétractions. La prise en charge thérapeutique du SDRC I est souvent délicate ; les traitements sont multiples et mal codifiés. Dans la littérature, seuls les biphosphonates par voies orale et intraveineuse ont prouvé leur efficacité . La calcitonine, les corticostéroïdes, la gabapentine, les vasodilatateurs et sympatholytiques ainsi que les blocs sympathiques stellaires et lombaires n’ont pas apporté de preuves scientifiques suffisantes pour être recommandées dans le traitement du SDRC I. Il en est de même pour la physiothérapie et l’ergothérapie .
L’utilisation de blocs nerveux périphériques (BNP) dans la prise en charge de raideurs articulaires liées au SDRC I est actuellement peu documentée. Les BNP semblent cependant avoir un intérêt à la fois diagnostique, en distinguant la part de douleur de celle de rétractions capsulo-ligamentaires dans la raideur articulaire, mais également thérapeutique, en permettant une kinésithérapie sous bloc grâce à l’analgésie obtenue. Les articles publiés à ce sujet sont peu nombreux et concernent uniquement des revues de cas .
Notre objectif est donc d’évaluer à la fois l’intérêt diagnostique et thérapeutique des BNP chez des patients présentant une raideur articulaire dans un contexte de SDRC I.
2.2
Méthodes
Il s’agit d’une étude rétrospective descriptive d’une série de cas concernant des patients présentant une raideur articulaire liées à un SDRC I et ayant bénéficié de blocs nerveux périphériques (BNP) dans le service de médecine physique et de réadaptation (MPR) du centre hospitalier universitaire (CHU) de Strasbourg entre janvier 2004 et janvier 2011.
2.2.1
Caractéristiques de la population
Nous avons recueilli les données cliniques, sociodémographiques et psychologiques de la population, les données en rapport avec le SDRC I et la raideur articulaire, et enfin celles en rapport avec le BNP proprement dit.
2.2.1.1
Données cliniques
Les patients présentaient un diagnostic de SDRC I avec raideur articulaire touchant une ou plusieurs articulation(s) d’un membre.
Le diagnostic de SDRC I était posé grâce à des critères cliniques (histoire de la maladie, douleur avec composante neuropathique, troubles vasomoteurs et/ou trophiques) associés ou non à une confirmation paraclinique par scintigraphie osseuse.
La raideur articulaire était mesurée soit par goniométrie standard, soit par la mise en évidence d’attitudes vicieuses.
Les critères d’exclusion étaient les suivants :
- •
allergie aux anesthésiques locaux (lidocaïne, ropivacaïne) ;
- •
troubles de la coagulation spontanés ou induits ;
- •
pathologie générale intercurrente évolutive ;
- •
infection cutanée au point de ponction ;
- •
coopération impossible.
Tous les patients ont signé un consentement libre et éclairé, après information claire et loyale.
2.2.1.2
Données sociodémographiques et psychologiques
Les données sociodémographiques recueillies ont été les suivantes : nombre de patients, sexe, âge, contexte socioprofessionnel (accident du travail, maladie professionnelle).
Les données psychologiques sont celles résultant d’une consultation psychiatrique demandée par le médecin MPR en cas de suspicion de troubles psychologiques.
2.2.1.3
Données en rapport avec le SDRC I et la raideur articulaire
Les données en rapport avec le SDRC I et la raideur articulaire ont été les suivantes :
- •
pathologie à l’origine du SDRC I ;
- •
durée d’évolution du SDRC I ;
- •
localisation de la raideur articulaire liée au SDRC I ;
- •
traitements antérieurs à la réalisation du BNP.
2.2.1.4
Données en rapport avec le BNP
Nous avons recueilli pour chaque BNP sa topographie : nerf(s) anesthésié(s) et voie d’abord.
2.2.2
Procédure
Les BNP ont été réalisés selon les principes de sécurité optimale, par un médecin anesthésique expert en anesthésie locorégionale du CHU de Strasbourg. Ils avaient lieu en salle de réveil, avec matériel de réanimation à proximité. Le patient était monitoré (électrocardiogramme, pression artérielle, saturation en oxygène) et une voie veineuse périphérique était posée. Le repérage du nerf à anesthésier ( Fig. 1 a et b) se faisait en utilisant les repères cutanés de surface (dessinés sur la peau grâce à un crayon dermographique), la neurostimulation (Stimuplex HSN12 ® BBraun, Allemagne ; intensité minimale de stimulation 0,4 mA ) avec aiguilles adaptées (Stimuplex ® BBraun pour blocs diagnostiques et Contiplex ® BBraun pour blocs continus thérapeutiques) et l’échographie [échographe LOGIQ e-ultrasound system (GE Healthcare, Chalfont Saint-Giles, Royaume Uni)]. Le médecin anesthésiste repérait le nerf et les structures environnantes, suivait en temps réel la progression de l’aiguille ainsi que la diffusion de l’anesthésique local. L’efficacité du BNP était systématiquement vérifiée grâce au test du glaçon (insensibilité au froid en cas de BNP efficace).