The Spleen and Lymph Nodes
Richard H. Sills
The spleen and lymph nodes are the major components of the mononuclear-phagocyte system (MPS), serving as a filter removing damaged cells and particulate matter, and delivering antigens to the immune system. The MPS, originally called the reticuloendothelial system, consists of fixed phagocytic cells in different organs. These cells share a common derivation from circulating blood monocytes. Functionally, these phagocytes interact locally with lymphocytes and play an essential role in the recognition of antigens and their interaction with immunocompetent cells. The MPS constitutes a crucial component of our immunologic defense mechanisms.
Although components of the MPS occur in most tissues, they are particularly dense in the spleen and lymph nodes. The specialized filtering capabilities of these organs provide ideal locations for contact between antigens and the immune system. Macrophages perching on endothelial cells and reticulum fibers assume the role of immunologic sentries and are vital in initiating host response.
THE SPLEEN
Anatomy
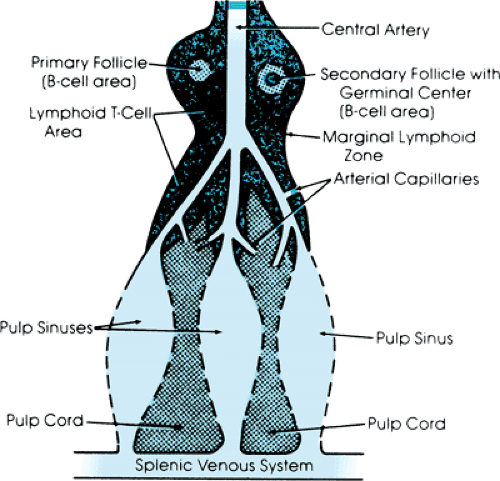
The spleen is the largest lymph node in the body. Its anatomy provides for uniquely close contact between its immunologic tissues and blood. The splenic tissue consists of red and white pulp lying within a capsule (Fig. 295.1). The white pulp, rich in T- and B-cell lymphocytes, is supplied by central arterioles. These vessels tend to branch at right angles, resulting in the preferable skimming of plasma into the white pulp for antigen processing. The main terminal splenic arteries, which contain the remaining hemoconcentrated blood, continue directly forward into the contiguous red pulp.
The red pulp is the majority of splenic tissue, consisting of splenic cords that interdigitate between splenic venous sinusoids. At least 90% of the hemoconcentrated blood reaching
the red pulp enters these splenic cords, which contain a fibrous network of mononuclear-phagocyte tissue. The circulation in the cords is designated as open because there is no well-defined endothelial lining. The cords lie between and share a basement membrane with the adjacent splenic venous sinuses. To exit the cords, blood must pass through 1- to 5-μm slits in this fenestrated basement membrane to reach the sinuses. The circulation through the cords is slow and congested because the blood reaching the red pulp is hemoconcentrated, and erythrocytes require additional time to pass through the small and limited number of slits that must be traversed to reach the sinuses. This delay provides prolonged exposure of blood cells, bacteria, and particulate matter to the dense mononuclear-phagocyte elements within the red pulp. This anatomic arrangement and the fact that the spleen (with an average weight of only 180 g in adults) receives approximately 6% of cardiac output provide a tremendous filtration capability.
the red pulp enters these splenic cords, which contain a fibrous network of mononuclear-phagocyte tissue. The circulation in the cords is designated as open because there is no well-defined endothelial lining. The cords lie between and share a basement membrane with the adjacent splenic venous sinuses. To exit the cords, blood must pass through 1- to 5-μm slits in this fenestrated basement membrane to reach the sinuses. The circulation through the cords is slow and congested because the blood reaching the red pulp is hemoconcentrated, and erythrocytes require additional time to pass through the small and limited number of slits that must be traversed to reach the sinuses. This delay provides prolonged exposure of blood cells, bacteria, and particulate matter to the dense mononuclear-phagocyte elements within the red pulp. This anatomic arrangement and the fact that the spleen (with an average weight of only 180 g in adults) receives approximately 6% of cardiac output provide a tremendous filtration capability.
After blood reaches the sinuses, it passes into the splenic venous system. Blood from the sinuses enters trabecular veins and eventually the hepatic portal vein; there are no valves in this system, which remains at the same pressure as the hepatic portal vein.
Physiology
Although none of the spleen’s individual cells is unique, its distinctive anatomic arrangement provides it with characteristic functional capabilities.
Resistance to Infection
The spleen plays a major role in the processing of small doses of intravenous particulate and polysaccharide antigens that reach it through its vascular supply. Splenic macrophages efficiently ingest these intravenous antigens and deliver them to the immunocompetent cells of the spleen for antibody production.
The spleen is also critical in clearing circulating bacteria. Bacteria coated with antibody or nonencapsulated bacteria in the absence of a specific antibody can be effectively removed in MPS tissues other than the spleen. However, the amorphous polysaccharide coat of encapsulated bacteria greatly impairs their clearance in the absence of antibody; only the spleen’s highly efficient phagocytic cords can clear these bacteria effectively. The splenic white pulp then processes these intravenous antigens to produce antibody that during subsequent exposures allows for efficient clearance by the remainder of the MPS.
Filtering of Formed Elements of Blood
Erythrocytes endure a slow passage through the hypoxic and acidotic environment of the cords and then squeeze through narrow slits into the sinusoids. Although healthy erythrocytes readily accomplish this, many aged and abnormal red cells remain behind to be ingested by the macrophages lining the cords. Abnormal cells, such as spherocytes, sickle cells, and antibody-coated erythrocytes or platelets (especially those with light coatings of IgG), are mainly cleared by the spleen. The splenic cords are also uniquely capable of removing erythrocytic inclusions, such as nuclear remnants (i.e., Howell-Jolly bodies) or precipitated globin (i.e., Heinz bodies), without destroying the cell.
Other Functions of the Spleen
Other splenic functions include remodeling of reticulocytes, hematopoiesis during early fetal development, and a reservoir function for platelets and plasma proteins such as factor VIII. Its function as a reservoir for erythrocytes is insignificant except in pathologic states such as hypersplenism.
Physical Examination of the Spleen
The spleen is best palpated by standing on the right side of the child and examining the left side of the abdomen with the right hand. The child should be examined in the supine or right lateral decubitus position with the knees up. Only light pressure should be used in small children because the spleen can easily be pushed out of the way without feeling its edge.
A palpable spleen is not unusual in normal children. A 1- to 2-cm spleen tip is palpable below the left costal margin in 30% of full-term neonates and in as many as 10% of normal children. Almost 3% of healthy college freshman have palpable spleens. The normal, palpable spleen tip is soft and nontender. A spleen tip enlarged beyond 1 to 2 cm should be considered abnormal.
The spleen can usually be differentiated from other left upper quadrant masses by the absence of overlying bowel and its movement with respiration. It may occasionally be confused with the left lobe of the liver or a left upper quadrant tumor such as Wilms tumor or neuroblastoma. In the presence of any doubt, ultrasonography can usually define the anatomy.
Excessive Splenic Function
Splenomegaly
Splenomegaly is the most frequent and important clinical problem involving the spleen. The most common causes of splenic enlargement are listed in Box 295.1. Splenomegaly in children usually results from hyperplasia of the MPS, which can be categorized as excessive antigenic stimulation, disorders of immunoregulation, or excessive destruction of abnormal blood cells. The spleen is rarely the primary site of disease, usually being affected by a systemic process involving lymphoid tissues.
Excessive antigenic stimulation is usually the result of infection, which causes most splenomegaly in children. Viral infections do this most frequently, and the associated splenomegaly is usually transient and only mild to moderate in severity. Although Epstein-Barr virus and cytomegalovirus are the best known viral agents to cause splenomegaly, the more routine viral illnesses of childhood cause it more frequently. A less common but important cause of splenic enlargement is acquired immune deficiency syndrome. Other common infectious causes include bacterial, protozoal, and fungal infections. In endemic areas, malaria and schistosomiasis are routine causes of splenomegaly. Concomitant generalized lymphadenopathy is common in many of these infectious causes.
Disorders that result in the effective destruction of blood cells or that affect immunoregulation are less common causes of splenomegaly.
Neoplastic disorders may also present with splenomegaly. One-half of children with acute lymphoblastic leukemia have splenomegaly, which also occurs in the lymphomas (both Hodgkin disease and non-Hodgkin lymphoma) and acute myeloblastic leukemia. Metastatic involvement of the spleen, which is uncommon in children, is most often caused by neuroblastoma. The spleen can also be infiltrated by histiocytes, a condition in children that usually is caused by Langerhans cell histiocytosis.
Impaired venous blood flow in the splenic or portal venous system can cause splenomegaly. The most common causes include cavernous transformation of the portal vein, hepatic cirrhosis, and congestive heart failure. Children with extrahepatic portal venous obstruction, such as cavernous transformation, often present with splenomegaly as the primary manifestation of their disease.
BOX 295.1. Causes of Splenomegaly in Children
Hyperplasia of the monocyte-phagocyte system
Excessive antigenic stimulation
Viral infections
Infectious mononucleosis
Cytomegalovirus
Acquired immunodeficiency syndrome
Bacterial infections
Septicemia
Endocarditis
Salmonella
Protozoal infections
Toxoplasmosis
Malaria
Fungal infections
Histoplasmosis
Disorders of immunoregulation
Juvenile rheumatoid arthritis
Systemic lupus erythematosus
Serum sickness
Excessive destruction of blood cells
Hereditary spherocytosis
Sickle cell disease
Neonatal Rh or ABO incompatibility
Neoplastic infiltration
Acute leukemias
Hodgkin disease
Non-Hodgkin lymphoma
Neuroblastoma
Histiocytoses
Benign tumors
Disordered splenic blood flow
Cavernous transformation of the portal vein
Hepatic cirrhosis
Congestive heart failure
Infiltration with abnormal material
Gaucher disease
Niemann-Pick disease
Space-occupying lesions
Hematomas
Pseudocysts
Congenital cysts
Extramedullary hematopoiesis
Thalassemia major
Osteopetrosis
Storage diseases such as Gaucher or Niemann-Pick disease are associated with splenomegaly because of the accumulation of abnormal lipids in splenic macrophages. Splenomegaly may be the first clinical manifestation of these disorders.
After trauma, palpable subcapsular hematomas may develop in the spleen. These hematomas may eventually develop into clinically palpable pseudocysts. Congenital splenic cysts usually present with asymptomatic splenomegaly.
Although normally only found during early fetal development, extramedullary hematopoiesis may occur in diseases associated with intense demand on the bone marrow for cell production. Thalassemia major and osteopetrosis are examples of this rare cause of splenomegaly.
Hypersplenism
Hypersplenism is a clinical syndrome in which splenic function becomes excessive as the spleen and its MPS tissues enlarge. It is defined by the following criteria: splenomegaly, a deficiency of at least one or more of the peripheral blood cell lines, normal or increased levels of bone marrow precursors, and an expectation that splenectomy will resolve the cytopenias. As the spleen enlarges, it can sequester erythrocytes, leucocytes, and platelets, resulting in decreases in some or all of these cell lines.
The most common cause of hypersplenism is venous obstruction. Because of the absence of valves in the portal venous system, an increase in portal pressure is reflected immediately in the splenic venous sinuses. This impairs blood flow out of the cords and results in the sequestration of blood cells and hypersplenism. Hypersplenism in children most often is caused by portal hypertension due to extrahepatic venous obstruction, often secondary to thrombosis of the portal vein caused by umbilical venous catheterization, septic omphalitis, or thrombosis because of dehydration or shock. Intrahepatic venous obstruction is usually due to cirrhosis. Schistosomiasis and malaria are important causes in endemic areas.
Children with hypersplenism can present with simple fatigue, pallor, and irritability or with unexplained splenomegaly. Portal hypertension increases flow through minor collateral vessels between the portal and systemic circulation. This can result in recognizable dilation of the superficial abdominal veins as well as esophageal varices. These varices may present with sudden and catastrophic gastrointestinal hemorrhage. Esophagoscopy is the most accurate means for confirming esophageal varices.
Therapy of hypersplenism depends on the site, nature, and severity of the vascular obstruction. Splenectomy cures the pancytopenia, but it is usually not indicated because the pancytopenia rarely causes serious problems. However, vascular shunts may be necessary to prevent esophageal variceal bleeding.
Hypersplenism occurs less frequently as a result of splenomegaly in the absence of venous obstruction, as in infections such as malaria and storage diseases such as Gaucher disease.
The splenic sequestration crisis is a distinct form of acute hypersplenism in young children with sickle cell disease. These children may develop sudden and massive splenic enlargement with sequestration of large portions of blood volume. They present with sudden weakness, dyspnea, left-sided abdominal pain, and increasing splenomegaly. So much blood can be trapped within the spleen that death due to hypovolemia can rapidly result. Treatment consists of restoration of blood volume and transfusion. To prevent recurrences, splenectomy may be indicated.
Impairment of Splenic Function
Causes
Hyposplenism may be anatomic (e.g., absence of splenic tissue), functional (e.g., impaired function despite an intact spleen), or a combination of both. The most common causes of hyposplenism in children are surgical splenectomy, sickle cell disease, and congenital absence of the spleen. The functional hyposplenism of sickle cell disease is readily understood in physiologic terms. The hypoxic and acidotic conditions in the splenic cords are ideal for inducing sickling. Once sickled, the erythrocytes are less able to pass from the cords to the sinuses. Splenic circulation becomes so obstructed that splenic function is gradually lost in most patients in the first 2 to 3 years of life.
Conditions associated with acquired hyposplenism include inflammatory bowel disease, systemic lupus erythematosus,
immune complex glomerulonephritis, splenic irradiation, graft-versus-host disease, and adult celiac disease. Normal neonates may demonstrate impaired splenic function as a developmental phenomenon, although its clinical significance is not known.
immune complex glomerulonephritis, splenic irradiation, graft-versus-host disease, and adult celiac disease. Normal neonates may demonstrate impaired splenic function as a developmental phenomenon, although its clinical significance is not known.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree