The Failed Arthroplasty: Options for Revision
Julie Y. Bishop
Evan L. Flatow
J. Y. Bishop: Clinical Shoulder Fellow, Department of Orthopaedic Surgery, Mount Sinai Medical Center, New York, New York.
E. L. Flatow: Lasker Professor of Orthopaedic Surgery, Chief of Shoulder Surgery, Department of Orthopaedic Surgery, Mount Sinai Medical Center, New York, New York.
INTRODUCTION
Revision shoulder arthroplasty is a demanding procedure that usually involves not only component revision but also complex reconstruction of soft tissue contracture, muscle deficiency, and bone loss. It is undertaken only after careful evaluation of the causes of failure, especially ensuring that infection is not the underlying cause. Meticulous planning is also essential for a favorable outcome. Although clinical results of revision shoulder arthroplasty are generally inferior to primary shoulder arthroplasty, careful patient selection and adherence to surgical principles can lead to satisfactory results.
EVALUATION
Several factors must be evaluated when considering revision surgery, including the patient factors and expectations, cause of the implant and/or clinical failure, careful review of the operative reports for any clues as to the cause of failure, integrity of the soft tissue and bony envelope, and ability to satisfactorily improve the patient’s condition. In many cases, the cause of primary arthroplasty failure is multifactorial, and thus several different reconstructive procedures may need to be performed during revision surgery. Thus, careful evaluation of the cause of failure, in particular, ensuring that the cause is not infection, is mandatory before attempting revision arthroplasty.
Patient Goals
When a patient presents with complaints after shoulder arthroplasty, it is important to identify the chief complaint. Although most patients will present with the general complaint of pain and loss of function, these problems must be separated if possible. It is important to understand what bothers patients the most and what they hope to gain from revision surgery. Are they primarily seeking pain relief, or do they have tolerable pain but are dissatisfied with a poor functional outcome? This is especially important in cases in which severe structural loss make it unlikely that major functional gains can be expected. Sometimes it can be helpful to play a thought experiment: The patient is asked which of two procedures he or she would choose: one that would relieve all of the pain but not improve motion or strength or one that would increase function but leave pain unaltered.
History
Although it can be long and tedious, it is important to go carefully through the patient’s history. The patient should always be asked about any history of infection or poor wound healing. The details of the postoperative rehabilitation may give clues as to any current stiffness. Understanding the onset of problems after the prior surgery may provide clues as to the cause of failure. Patients with an acute postoperative infection or instability commonly present with ongoing pain without improvement after surgery. Patients with delayed infection, glenoid arthrosis,
or aseptic loosening more commonly present with a period of pain relief followed by a new onset of symptoms.
or aseptic loosening more commonly present with a period of pain relief followed by a new onset of symptoms.
All prior operative reports must be obtained and reviewed. A surgeon should not make a final evaluation, and certainly not undertake revision surgery, without this important piece of information. When a primary arthroplasty fails, the evaluating surgeon should think of himself or herself as a detective. No stone should be left unturned, and every possible reason for failure should be examined. Thus, the prior operative report is an essential “clue” as to the cause of failure. It contains information that most patients may not know or understand and thus cannot articulate to the evaluating surgeon. Any complications, especially any neurovascular abnormalities or complications, should be noted. Most important, the type and size of the prosthetic implant should be noted in the report. In addition, every effort should be made to obtain the previous radiographs, office notes of clinical follow-up, and postoperative rehabilitation program. It can be very helpful, if the patient gives permission, to speak with the original surgeon. Much useful information, not in the written record, may be obtained, particularly about the patient’s personality and adherence to postoperative instructions.
Physical Examination
The physical examination begins with inspection for surgical scars, abnormal posture, and muscular atrophy. Active and passive range of motion of the shoulder are assessed, and if any loss of motion is detected one should attempt to determine the cause of motion loss (i.e., whether by soft tissue contracture, bony impingement, or prosthetic impingement). The strength of the shoulder and upper extremity should be assessed with particular attention to rotator cuff and deltoid function. The stability of the joint is examined, documenting the degree and direction of any instability. A click or clunk during range of motion or instability examination may be indicative of a loose glenoid component. Careful attention should be paid to which movements and positions elicit pain as well. Finally, a neurologic examination of the upper extremity should be performed and investigated with electrodiagnostic studies if a deficit is suspected.
Imaging
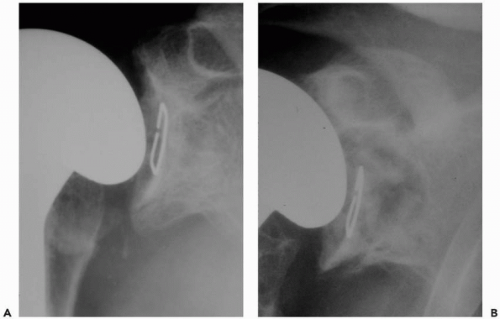
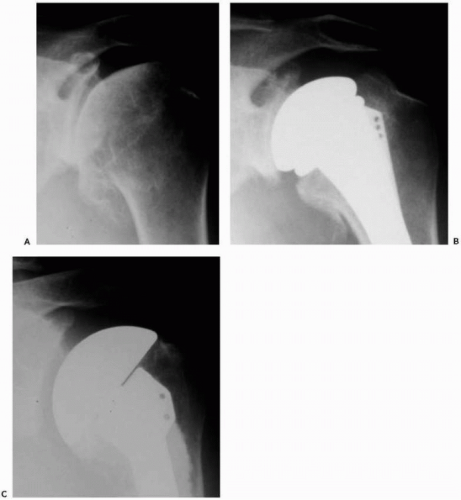
A standard radiographic series should be performed in all patients to include anteroposterior views of the shoulder and glenohumeral joint, a lateral “Y” scapular view, and an axillary view. If possible, serial radiographs including preoperative and postoperative x-rays should be assessed for radiolucent lines, osteolysis, prosthetic position, and prosthetic migration (Fig. 28-1). If glenoid or humeral loosening is suspected, fluoroscopically guided radiographs may aid in demonstrating radiolucent lines at the bone-cement interface.68
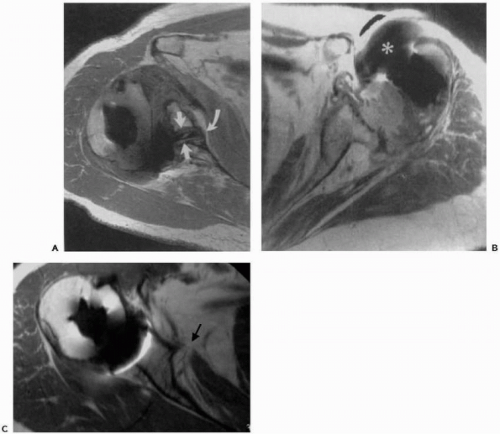
Although not necessarily a prerequisite to surgery, cross-section imaging, that is, computed tomography and magnetic resonance imaging (MRI), may provide important information on implant positioning, humeral and glenoid bone loss, glenoid version, remaining glenoid bone stock, and posterior glenoid erosion47,53 (Fig. 28-2, A and B). Determination of overall bony losses and bone stock should be done before surgery and should not be a surprise intraoperative finding for which one is unprepared to handle. Finally, limited pulse-sequence parameter modification makes it possible for MRI to provide information on the status of the rotator cuff and residual cartilage despite the presence of an implant103 (Fig. 28-2C).
Infection
Infection should always be ruled out before embarking on revision surgery. This starts with a careful history, specifically asking about any postoperative wound complications, fever, chills, remote site of any infections, or any recent invasive procedures (e.g., colonoscopy and cystoscopy), all of which should raise suspicions of a prosthetic infection. Careful inspection of the wound and surrounding skin should be performed, looking in particular for any evidence of draining sinuses, persistent erythema, warmth, and lymphadenopathy.
One should routinely order a white blood cell count, erythrocyte sedimentation rate, and C-reactive protein as
general measures of inflammation/infection. If infection is suspected, an indium-labeled white blood cell scan is ordered, the joint is aspirated, and cultures for gram-positive bacteria, gram-negative bacteria, acid fast bacilli and fungi are obtained.
general measures of inflammation/infection. If infection is suspected, an indium-labeled white blood cell scan is ordered, the joint is aspirated, and cultures for gram-positive bacteria, gram-negative bacteria, acid fast bacilli and fungi are obtained.
INDICATIONS FOR REVISION
Patient Goals and Characteristics
Pain relief is the primary indication for revision shoulder arthroplasty. Although joint motion, strength, and function may also be achieved, the results after revision shoulder arthroplasty are more variable.3,28,33,34,58,60,66,80,87,88,92, 113,114 It is important during evaluation that patient disability and expectations are adequately assessed. In patients with minimal pain and disability despite a “failed” shoulder arthroplasty, nonoperative management may provide a satisfactory outcome. Similarly, elderly or medically compromised patients may have limited functional demands or be unfit for surgery. In these patients, nonoperative treatment including physical therapy, anti-inflammatory medications, and bracing (e.g., sling) may provide some benefit.
In patients with severe pain and disability who are good surgical candidates and have a complete understanding of the risks of surgical intervention, the prolonged rehabilitation program and the potential result of surgery, revision shoulder arthroplasty, should be considered. Specific indications for surgery include joint contractures and adhesions, joint instability, component malposition, component loosening, and/or wear, infection, and fracture.
Anatomic Factors
During revision shoulder arthroplasty, a number of anatomic lesions may coexist and contribute to the complexity of surgery. For example, prosthetic instability may be secondary to a combination of factors including contracture in the opposite direction of instability, soft tissue deficiency in the direction of instability, and component malposition. Further, component loosening and wear may contribute to osteolysis and bone loss. As already stated, the degree of bony loss should be predetermined, because this can affect the feasibility of implanting or revising a component, especially the glenoid. Knowing the degree of bony and/or soft tissue deficits preoperatively is essential to preoperative planning and certainly affects what can and cannot be done in the revision situation. Thus, before embarking on revision total shoulder arthroplasty it is important to construct a careful preoperative plan to ensure that the proper personnel and equipment are available. Intraoperatively, all pathologic findings including adhesions and contractures, soft tissue loss, bone loss, component malpositioning, component loosening/wear, and instability should be addressed to ensure a satisfactory outcome. Contraindications to revision shoulder arthroplasty are similar to contraindications to primary shoulder arthroplasty and include active infection, significant neurologic impairment, and inability to participate in a prolonged rehabilitation program, and patients whose medical or mental status precludes surgery.
PREPARATION FOR REVISION
Operative Planning
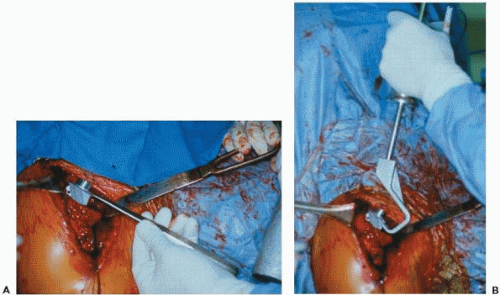
Careful and thorough preparation for the revision arthroplasty is essential because these cases can be long and arduous even for the most prepared of surgeons. The revision arthroplasty surgeon will be challenged enough by the complexity of the surgery itself, with no need to add the additional hardship of being unprepared. Before the case, all prior operative reports should be reread and any essential details about the anatomy, vascularity, and components noted. The current prosthesis should be exactly identified, and the proper personnel should be notified to bring any essential equipment. This includes proper extractors for the specific prosthesis and a universal extractor (Fig. 28-3). A long-stem prosthesis should be available in case of fracture, as well as cerclage wires or cables and other fixation equipment. If glenoid arthrosis is likely the problem and there is a chance that the humeral component will be retained, then the proper matching glenoid system should be available even if it is not the one routinely used by the operative surgeon. Different sizes of humeral heads should be available as well if only the humeral head, and not the entire prosthesis, needs to be changed. However, one should be prepared to remove the entire humeral prosthesis. Thus, in addition to the proper prosthesis-specific extractors, cement removal equipment must be available if the primary implant was cemented. This includes ultrasonic cement removal devices, high-speed low-torque drills, and cement removal instruments such as osteotomes and chisels.
Bony deficits are a common problem in the revision surgery and were explored in more detail in the previous chapter. However, in the planning stages, if one expects large humeral deficits or large segmental losses, bulk allograft should always be available. For glenoid bony losses, usually a small amount of allograft such as a femoral head allograft is typically enough, or if preferable, an iliac autograft may be planned.
Standard radiographs can be taken intraoperatively, but C-arm image intensification has proven to be very useful in evaluating component positioning, version, height, bone stock, and any possible fractures after component insertion or removal. Furthermore, image intensification can be used to verify the intrashaft position of high-speed tools used to
remove cement safely. In the revision situation, even the most prepared surgeon can run into difficulties with neurovascular structures, whether because of abnormalities, extensive scarring, or damage from the prior surgery, which cannot always be ascertained preoperatively. Therefore, it is wise to have a trained microvascular surgeon available during difficult cases.
remove cement safely. In the revision situation, even the most prepared surgeon can run into difficulties with neurovascular structures, whether because of abnormalities, extensive scarring, or damage from the prior surgery, which cannot always be ascertained preoperatively. Therefore, it is wise to have a trained microvascular surgeon available during difficult cases.
THE STIFF ARTHROPLASTY
Postoperative stiffness can be a common cause of failure of the primary shoulder arthroplasty and is a difficult and complex problem. The stiffness can be caused by inadequate release of contracted tissues at the time of the primary arthroplasty, extensive postoperative scarring because of an intense inflammatory healing response, or slow rehabilitation. Extra-articular and intra-articular adhesions may contribute to the loss of motion.
When surgical release is used to treat the stiff arthroplasty, early range-of-motion exercises and physical therapy are crucial for maintaining the increased motion that was obtained intraoperatively.57,77,79,85,111 Using a postoperative interscalene catheter for continuous regional block may be helpful in obtaining early postoperative pain-free range of motion.30 When postoperative pain, which can be extensive, is managed with either intravenous or oral narcotics, doses are often high, and even if pain is controlled, patients are typically too sleepy or nauseated to participate in physical therapy. When used in conjunction with interscalene regional anesthesia, the patient can avoid general anesthesia altogether, obtains excellent postoperative pain control, and subsequently is awake and alert and can start physical therapy the same day as surgery.30 There are also psychologic benefits to using an interscalene catheter because the patient can immediately see the motion gains obtained during surgery. The patient can then see and believe that this motion is possible and can be achieved. Also, patients are more apt to participate willingly in physical therapy when the initial sessions are less painful.9,22 The catheter provides excellent pain relief, is safe, and has been found to have a high rate of patient satisfaction as well.30 However, an infusion rate that retains protective sensation is advisable, lest tendon repairs be compromised by a temporarily “insensate” joint.
Technique of Release
Although the senior author does not routinely perform shoulder arthroscopy before open revision shoulder arthroplasty, shoulder arthroscopy may be beneficial in patients with stiffness caused by capsular contracture (in which capsular release is performed).58,108 However, when extra-articular scarring is present, between the subscapularis, strap muscles, deltoid, and pectoralis, arthroscopic release alone is inadequate and a formal open release is necessary.
Arthroscopy also allows the identification and possible treatment of any associated pathology. It may also guide the surgeon toward an open approach if more significant problems, such as glenoid loosening, are found as well.
Arthroscopy also allows the identification and possible treatment of any associated pathology. It may also guide the surgeon toward an open approach if more significant problems, such as glenoid loosening, are found as well.
When an open revision arthroplasty for the stiff shoulder is carried out, extensive releases are performed (Fig. 28-4). After the standard deltopectoral exposure, which may be extended for the revision surgery, subdeltoid and subacromial adhesions are released and/or resected first. The coracohumeral ligament is released as well because it is often contracted in the revision cases. These structures must all be meticulously released to restore soft tissue mobility. The subscapularis and biceps tendons are then identified, and the axillary nerve is palpated and identified at the lower border of the subscapularis tendon. The axillary nerve can usually be identified approximately 3 to 5 mm medial to the musculotendinous junction.
In revision cases, this relationship may be distorted and the axillary nerve is often scarred and difficult to identify. The “tug test” has proved to be useful to confirm the identity of the axillary nerve. The “tug test” is performed by placing a finger under the deltoid on the anterior branch of the axillary nerve and another finger from the opposite hand on what one thinks is the axillary nerve as it passes inferior to the subscapularis tendon.39 As one gently presses on the nerve with one finger, the force is transmitted through the axillary nerve and felt in the opposite finger, confirming the identity of the axillary nerve. After identification, the axillary nerve is protected and periodically reexamined throughout the procedure. When there is a preoperative suspicion of nerve injury, or a high degree of scarring precluding simple identification of the nerve, the assistance of a microsurgeon to locate and free the nerve (and plexus as needed) may be helpful.
The subscapularis tendon is then thoroughly inspected for its continuity and quality. In revision cases, the subscapularis tendon is commonly scarred and contracted and limits external rotation of the shoulder. The subscapularis tendon, along with the underlying capsule, is then sharply released from its insertion on the lesser tuberosity. If bone quality allows, the insertion of the subscapularis is released with a thin wafer of bone using an osteotome (Fig. 28-5). This permits bone-bone healing after repair.
The subscapularis is then tagged, and a release of the subscapularis is performed by releasing the superior structures (i.e., rotator interval, coracohumeral ligament), anterior structures (i.e., adhesions between the subscapularis and subcoracoid space/strap muscles), inferior structures (i.e., adhesions between subscapularis and inferior capsule/axillary nerve), and posterior structures (i.e., anterior capsule) (Fig. 28-6).
To release the anterior capsule from the subscapularis tendon, a plane is initially bluntly developed between the anterior capsule and subscapularis. This is then sharply released, restoring the excursion of the subscapularis tendon. A complete capsular release is confirmed when the normal elastic feeling (or bounce) of the subscapularis tendon is restored when pulling on the traction sutures. Care is taken to avoid the subscapularis innervation medially.
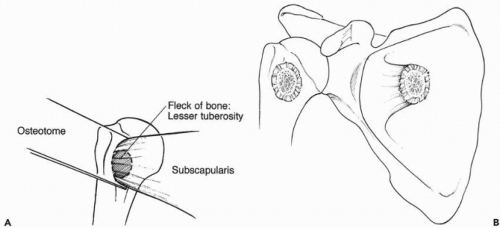 Figure 28-5 (A,B) Small piece of lesser tuberosity is taken with the subscapularis during the release from the humerus. This allows bone-to-bone healing. |
A complete subscapularis release is usually all that is required to restore the normal soft-tissue tension of the anterior structures. If external rotation is still limited, then a medial transfer of the subscapularis insertion may be performed by repairing the subscapularis tendon to the humeral osteotomy at the completion of the procedure. This effectively further lengthens the subscapularis tendon. To further lengthen the subscapularis, a coronal Z-plasty lengthening has been described.87,91 However, in revision cases the subscapularis is often atrophic and tissue quality is inadequate to permit a safe Z-plasty lengthening, unless the subscapularis was previously shortened (Putti-Platt).
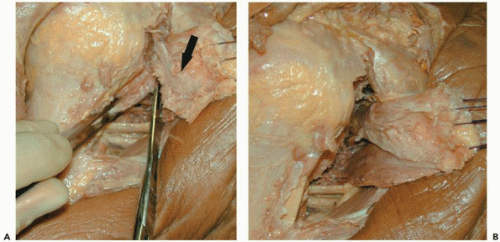 Figure 28-6 (A) Cadaveric example of technique for resection of the capsule from the undersurface of the subscapularis (black arrow). (B) Improved mobilization of the subscapularis noted. |
After any necessary work is performed on the humerus, attention is turned toward the posterior inferior capsule. Exposure of the glenoid also provides access to this aspect of the capsule, which must be assessed and released in the stiff shoulder. A Fukuda ring retractor or bone hook is used to retract the humerus away from the glenoid, and a spiked, curved retractor is used to expose the anterior glenoid margin (Fig. 28-4B). With the inferior capsule exposed and
axillary nerve protected, the inferior capsule is then incised with electrocautery. It is important during this procedure to place the arm in adduction and internal rotation, which allows the nerve to fall away from the glenoid rim. If the patient is not paralyzed, incising the capsule with electrocautery and observing for deltoid muscle contraction during release is also an indicator of nerve proximity. Similarly, the superior capsule is sharply dissected off the supraspinatus tendon, and the capsule is released.
axillary nerve protected, the inferior capsule is then incised with electrocautery. It is important during this procedure to place the arm in adduction and internal rotation, which allows the nerve to fall away from the glenoid rim. If the patient is not paralyzed, incising the capsule with electrocautery and observing for deltoid muscle contraction during release is also an indicator of nerve proximity. Similarly, the superior capsule is sharply dissected off the supraspinatus tendon, and the capsule is released.
With the humerus retracted posterior and lateral, the posterior capsule can now be evaluated. In most cases the posterior capsule will not be contracted but may be capacious secondary to posterior humeral subluxation. In this instance, inadvertently releasing the capsule can predispose to postoperative posterior instability. In general, posterior capsular redundancy is treated by releasing the tight anterior structures (i.e., 360-degree subscapularis release), effectively balancing the shoulder. However, in the stiff shoulder, posterior contracture may be present, and a final assessment can be made with the trial components in situ. At this point, any capsular contracture may be corrected with release, because severe stiffness may require global capsular release.
Rehabilitation
The postoperative management of patients after revision shoulder arthroplasty performed for stiffness is tailored specifically to the diagnosis and operative procedure. In this instance, immediate motion and stretching are instituted as the interscalene catheter allows pain-free motion. The only restriction is that external rotation is usually limited to 45 degrees for the first 6 weeks to protect the subscapularis repair. Patients remain in the hospital for 2 to 3 days for vigorous supervised physical therapy while the interscalene catheter provides pain relief. Patients must understand the importance of their postoperative rehabilitation program. Surgical release of the contracted tissues is only half of the battle, and all patients should be committed to their rehabilitation and understand their role in obtaining a successful outcome. After the patient is discharged from the hospital, he or she should have a supervised physical therapy session five times per week for the first 2 weeks, which is then reduced to three times per week. In addition, patients should perform the stretches they learn on their own four times per day. The therapy should be focused on vigorous stretches and range of motion in all planes, using pulleys, external rotation with a stick, and so forth. If patients are not diligent in their therapy, all the initial gains made in range of motion will be quickly lost.
THE UNSTABLE ARTHROPLASTY
Instability is one of the more common complications after shoulder arthroplasty with the reported prevalence ranging from 0% to 29%, with an overall average in the literature reported in one study at 2.8%.37,38,50,75,90,106,112,115 Causes include bony insufficiency, malposition of components, and soft tissue deficiency. Once instability is addressed and corrected at surgery, a careful postoperative rehabilitation plan is critical to success.
Glenoid and Humeral Version
Excessive retroversion of the glenoid component with accompanying posterior instability is commonly a result of uncorrected posterior glenoid erosion. This is especially prevalent after arthroplasty for postcapsulorraphy arthritis, chronic posterior dislocations, or severe osteoarthritis. Humeral component malpositioning is less likely as a cause of instability, although it can occur50,60,61,64,78,114 (Fig. 28-7). Anterior glenoid insufficiency is less common but may be seen in patients with chronic anterior dislocations or rheumatoid arthritis, or after glenoid fractures. Failure to correct this with placement of the glenoid in anteversion can lead to anterior instability.
Preoperative assessment of component position requires careful radiographs, although axillary radiographs may be misleading as to glenoid component version. Image intensification can be used to profile the humeral component, and simultaneous inspection of the forearm position allows estimation of humeral version. However, magnetic resonance scanning with special sequences is likely the most reliable imaging option for glenoid component version102 (Fig. 28-8).
Intraoperative assessment at revision surgery of the glenoid component version requires adequate visualization. All overlying soft tissues and osteophytes are removed from the glenoid rim, allowing adequate exposure of the glenoid. By palpating the anterior glenoid neck and comparing intraoperative findings to preoperative imaging, one can estimate the version of the glenoid.
If there is substantial malposition of a well-fixed glenoid component, the component must be revised. Removing a solidly fixed glenoid component can be performed by first separating the glenoid face from the keel (or pegs) by using a sharp osteotome and then by removing the keel (or pegs) with osteotomes or a high-speed burr. If the native glenoid or remaining glenoid after component removal is maloriented (usually retroverted), then the prominent side (usually anterior rim) may be lowered with eccentric reaming. One must avoid the temptation of supporting a new glenoid component with asymmetric buildup of cement beneath the glenoid component. Over time, this will lead to cement fragmentation and glenoid component loosening. Eccentric reaming is performed by first initially lowering the high side using a high-speed burr and then drilling a centering hole to accommodate the glenoid reamer. The final glenoid version is then adjusted by reaming the “high” side until a concentric glenoid is established in the appropriate version (Fig. 28-9). Because the glenoid vault becomes shallow and narrow as one reams medially, high-side resection should be
limited to less than 1 cm. Also, at least some of the subchondral plate should be retained to support the component. If resection greater than 1 cm is required or cancellous bone starts to become exposed, mild retroversion (up to 10-15 degrees) may be accepted with a compensatory decrease in the retroversion of the humeral component. If there is less than 1 cm of glenoid bone stock available, the senior author does not resurface the glenoid.
limited to less than 1 cm. Also, at least some of the subchondral plate should be retained to support the component. If resection greater than 1 cm is required or cancellous bone starts to become exposed, mild retroversion (up to 10-15 degrees) may be accepted with a compensatory decrease in the retroversion of the humeral component. If there is less than 1 cm of glenoid bone stock available, the senior author does not resurface the glenoid.
In rare cases, the version cannot be corrected completely by eccentric reaming, and then bone grafting can be performed.62,81,104 In revision cases, an iliac crest or allograft is usually required. In this situation, a contoured graft is initially held in place by temporary K-wire fixation and then definitively fixed with two 3.5-mm cortical screws. When placing the screws, one should take care to avoid interfering with the eventual position of the glenoid component. The finer details of bone grafting and assessing bony deficiency are covered in more depth in the chapter on bony deficits.
Soft Tissue Insufficiency and Difficulties
In some revision cases the subscapularis tendon may be absent or the tissue inadequate to permit a functional anterior soft tissue repair. These cases are commonly associated with
anterior instability.50,61,64,78,112 Moeckel et al.78 reported on the findings and results of reoperation in seven patients who developed anterior instability after arthroplasty. All seven were found to have a disruption of the sutured subscapularis tendon, which was then repaired. Three patients had a recurrence of the anterior instability, which was subsequently addressed with a bone-Achilles tendon allograft. In addition to allograft, for subscapularis tendon insufficiency, a pectoralis major transfer can also be performed to replace the function of the subscapularis tendon.94,116 Although several variations are possible, the senior author prefers to transfer the sternocostal head of the pectoralis major tendon deep to the conjoined tendon, accurately reproducing the orientation of the subscapularis tendon.71 In addition, transferring the sternocostal head superficial to the musculocutaneous nerve (but deep to the conjoint tendon) decreases the tension on the musculocutaneous nerve after transfer.71 This minimizes the potential risk of nerve injury. During transfer it is important to carefully identify and visualize the musculocutaneous nerve to ensure that excessive tension is not created in the nerve. Occasionally, the proximal branches of the musculocutaneous nerve must be released to decrease the tension in the musculocutaneous nerve proper.71 In some cases, altering of the humeral component version may also be necessary. A slight increase in the humeral component retroversion may help to prevent anterior dislocation. Anterior placement of the humeral head on the shaft or an oversized component can further stress the anterior stability as well as the subscapularis repair. Rotating an offset humeral head to a more posterior position can help avoid anterior instability to some extent.
anterior instability.50,61,64,78,112 Moeckel et al.78 reported on the findings and results of reoperation in seven patients who developed anterior instability after arthroplasty. All seven were found to have a disruption of the sutured subscapularis tendon, which was then repaired. Three patients had a recurrence of the anterior instability, which was subsequently addressed with a bone-Achilles tendon allograft. In addition to allograft, for subscapularis tendon insufficiency, a pectoralis major transfer can also be performed to replace the function of the subscapularis tendon.94,116 Although several variations are possible, the senior author prefers to transfer the sternocostal head of the pectoralis major tendon deep to the conjoined tendon, accurately reproducing the orientation of the subscapularis tendon.71 In addition, transferring the sternocostal head superficial to the musculocutaneous nerve (but deep to the conjoint tendon) decreases the tension on the musculocutaneous nerve after transfer.71 This minimizes the potential risk of nerve injury. During transfer it is important to carefully identify and visualize the musculocutaneous nerve to ensure that excessive tension is not created in the nerve. Occasionally, the proximal branches of the musculocutaneous nerve must be released to decrease the tension in the musculocutaneous nerve proper.71 In some cases, altering of the humeral component version may also be necessary. A slight increase in the humeral component retroversion may help to prevent anterior dislocation. Anterior placement of the humeral head on the shaft or an oversized component can further stress the anterior stability as well as the subscapularis repair. Rotating an offset humeral head to a more posterior position can help avoid anterior instability to some extent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree