Tailoring Therapy to Individual Patients
Paul D. Miller
The pharmacological agents for the prevention and treatment of postmenopausal osteoporosis (PMO) registered with the Food and Drug Administration (FDA) are listed in Table 9.1.
The FDA has distinguished between prevention and treatment registrations for PMO. In order to gain a prevention indication, an agent needs to show that bone mineral density (BMD) loss can be prevented in early postmenopausal women as compared with placebo. For a treatment indication, an agent must show evidence of significant vertebral fracture risk reduction over a 3-year period as compared with placebo. Hence, there are agents that have FDA labels for both indications, and agents that have evidence for only a prevention indication [e.g., hormonal replacement therapy (HT)].
It is also important to put the HT registration in historical perspective: When HT was FDA approved for PMO, the “prevention vs. treatment” registration distinction did not exist. The treatment (e.g., fracture) distinction was created by the FDA when the evidence became available that sodium fluoride increased BMD in a linear manner yet was not associated with any reduction in vertebral fracture risk [1]. In fact, in the U.S. fluoride study just cited, nonvertebral fracture events were greater in the treated (fluoride) group than placebo. Due to this disconnect between improvements in BMD and lack of risk reduction, the FDA changed the registration requirements for a “treatment” indication to fracture risk reduction as opposed to maintenance of BMD. However, the doses of fluoride used in the pivotal U.S. fluoride study induced a very abnormal bone histology, which explained the poor bone quality and strength despite improvements in BMD. Nevertheless, requirements for registration for osteoporosis agents now had two categories: prevention and treatment. Notwithstanding, data from the large, prospective Women’s Health Initiative (WHI) study [2] were the
first to show evidence for significant reduction in both vertebral as well as nonvertebral fracture risk. However, to gain a “new” label, the end point must be the primary end point, and in the WHI dataset, fracture risk assessment was a secondary end point. Hence, there is still no FDA label for HT for “treatment.” In a similar manner, cyclical administration of the first-generation nonamino bisphosphonate etidronate did not gain FDA registration for “treatment,” since the FDA treatment requirement is evidence over 3 years of significant vertebral fracture risk reduction. In the pivotal clinical trials, etidronate did achieve significant fracture risk reduction over 2 but not through 3 years, as compared with placebo [3]. Etidronate registration got “caught” in the FDA registration requirement change (BMD to fracture) as a result of the fluoride studies, since when the etidronate clinical trials were planned, the FDA requirement for registration was a BMD, not fracture, end point. The etidronate clinical trials were statistically powered for a BMD, not a fracture, end point (e.g., smaller sample sizes). Etidronate thus never gained FDA registration for the treatment of PMO, due in larger part to a change in the registration data requirement. Nevertheless, cyclical etidronate is registered in more than 30 other countries for the treatment of PMO and still is used for the treatment of PMO in the United States in specific circumstances (e.g., intolerability to newer amino bisphosphonates).
first to show evidence for significant reduction in both vertebral as well as nonvertebral fracture risk. However, to gain a “new” label, the end point must be the primary end point, and in the WHI dataset, fracture risk assessment was a secondary end point. Hence, there is still no FDA label for HT for “treatment.” In a similar manner, cyclical administration of the first-generation nonamino bisphosphonate etidronate did not gain FDA registration for “treatment,” since the FDA treatment requirement is evidence over 3 years of significant vertebral fracture risk reduction. In the pivotal clinical trials, etidronate did achieve significant fracture risk reduction over 2 but not through 3 years, as compared with placebo [3]. Etidronate registration got “caught” in the FDA registration requirement change (BMD to fracture) as a result of the fluoride studies, since when the etidronate clinical trials were planned, the FDA requirement for registration was a BMD, not fracture, end point. The etidronate clinical trials were statistically powered for a BMD, not a fracture, end point (e.g., smaller sample sizes). Etidronate thus never gained FDA registration for the treatment of PMO, due in larger part to a change in the registration data requirement. Nevertheless, cyclical etidronate is registered in more than 30 other countries for the treatment of PMO and still is used for the treatment of PMO in the United States in specific circumstances (e.g., intolerability to newer amino bisphosphonates).
Table 9.1. FDA-approved medications for use in osteoporosis | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
Table 9.1 shows the FDA-registered therapies for PMO at the current time. Nasal or injectable calcitonin, raloxifene, alendronate, risedronate, ibandronate, and teriparatide are registered for the treatment of PMO [4,5,6,7,8,9,10,11,12,13]. This chapter will focus on these registered products, recognizing that physicians also utilize in an “off-label” fashion intravenous pamidronate, zolendronic acid, and HT for treatment (now defined as risk reduction) in individual patients for particular clinical reasons. In that vein, strontium ranelate (Protelos), registered in Europe for treatment of PMO, may also become available for off-label use in the United States, by patients obtaining access via other nations’ pharmacy availability [14]. In addition, the 1–84 parathyroid hormone (PTH; PREOS) formulation was recently also approved for the treatment of PMO in Europe and is under consideration for registration in the United States [15].
Individual treatment decisions for selecting therapy for PMO are complex. Although there are “guidelines” published for whom to treat and when to treat to prevent BMD loss or to reduce the risk of fracture by specific highly acknowledged organizations [National Osteoporosis Foundation (NOF), American Association for Clinical Endocrinologists (AACE)], treatment decisions are often individualized by clinicians based on case-by-case individual circumstances [16,17]. Although important health-economic considerations for treatment “thresholds” based on public-policy considerations are critical to putting the costs of osteoporosis treatment in the proper perspective of global health-economics and competition for public expenditures for many other chronic diseases, the physician and patient embark on decisions often based on what an individual patient needs. So, although the important considerations for health-economic-based decisions are very necessary, this chapter will try to incorporate the issues of evidence of efficacy of therapies with opinion-based decisions based on broad clinical experience.
Patients with Prior Fragility Fractures
Postmenopausal women with prior fragility fracture are at high risk for future fracture [18,19,20,21,22]. These patients are at high risk for future fractures within a short period of time after a fracture—even at skeletal sites that are distant from where the original fracture occurred (Table 9.2) [22]. These fragility fractures are predictive of future fracture risk, independent of the prevailing BMD (or T-score) [23]. Exactly why a prior fragility fracture at one skeletal site conveys a high risk for a future fracture at a distant skeletal site is unclear. Perhaps, in the untreated postmenopausal population, a fragility fracture is symbolic of systemic skeletal fragility. In those patients with hip fractures, evidence indicates that the older the patient, the less important low BMD becomes as a contributor for the hip fracture, and that falls are a larger component of the risk [24]. In the National Osteoporosis Risk Assessment (NORA) dataset, even in those women who have reported a prior wrist fracture after the age of 45 years, there is a greater risk for all (global) fractures at nonwrist skeletal sites [25]. Even though a solid reason for why there is a greater risk for a second fracture following the first fracture is not definitive, the reality is that it is—and these are patients who need the strongest considerations for treatment interventions.
Table 9.2. Prior fractures as a predictor | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
One of the limitations of the pharmacological clinical trials that have shown evidence for fracture risk reduction in postmenopausal women is
that the patients randomized between treatment and placebo groups have been randomized based on a prior vertebral fracture and/or a low BMD (e.g., T-score of – 2.0 or below) and are 60 years of age or older. Though a prior fragility fracture of the forearm, hip, or shoulder conveys a high risk for future fracture, it is unknown if treatment with the FDA-approved osteoporosis-specific pharmacological agents reduces the risk in the other postmenopausal populations who have higher BMD levels or who are under the age of 60 years. Nevertheless, clinicians should seriously consider treatment intervention beyond adequate vitamin D and calcium in postmenopausal women who sustain a fragility fracture after the age of 45 to 50 years. The exception is the data from the Women’s Health Initiative (WHI), which did show a benefit of HT in reducing fractures in a population generally considered not to have had osteoporosis by World Health Organization (WHO) criteria and in subgroups of the WHI under the age of 60 years [26].
that the patients randomized between treatment and placebo groups have been randomized based on a prior vertebral fracture and/or a low BMD (e.g., T-score of – 2.0 or below) and are 60 years of age or older. Though a prior fragility fracture of the forearm, hip, or shoulder conveys a high risk for future fracture, it is unknown if treatment with the FDA-approved osteoporosis-specific pharmacological agents reduces the risk in the other postmenopausal populations who have higher BMD levels or who are under the age of 60 years. Nevertheless, clinicians should seriously consider treatment intervention beyond adequate vitamin D and calcium in postmenopausal women who sustain a fragility fracture after the age of 45 to 50 years. The exception is the data from the Women’s Health Initiative (WHI), which did show a benefit of HT in reducing fractures in a population generally considered not to have had osteoporosis by World Health Organization (WHO) criteria and in subgroups of the WHI under the age of 60 years [26].
Postmenopausal Patients With Low Bone Mineral Density Without Prior Fracture
Patients who have “osteoporosis” or “osteopenia” by WHO criteria are at increased risk for fragility fractures—based simply on their low BMD alone. In fact, whether the technique for measuring BMD uses central or peripheral bone mass measuring devices, there are a greater number of postmenopausal
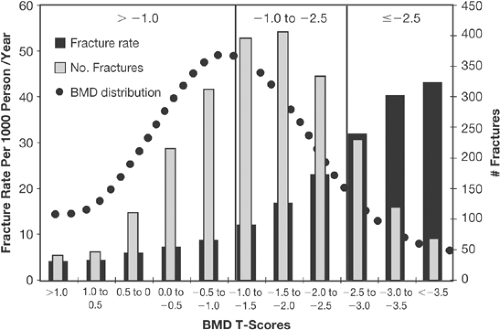
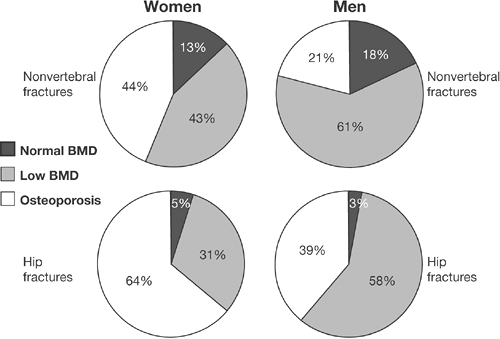
women who sustain fractures that have WHO “osteopenia” rather than osteoporosis—because there are simply more people with osteopenia than osteoporosis [27,28,29] (Figs. 9.1 and 9.2). The latter observations present a conundrum—how to stratify risk to select those patients who are at greater risk for fracture who do not have WHO osteoporosis without overtreating those with osteopenia that have a lower fracture risk.
women who sustain fractures that have WHO “osteopenia” rather than osteoporosis—because there are simply more people with osteopenia than osteoporosis [27,28,29] (Figs. 9.1 and 9.2). The latter observations present a conundrum—how to stratify risk to select those patients who are at greater risk for fracture who do not have WHO osteoporosis without overtreating those with osteopenia that have a lower fracture risk.
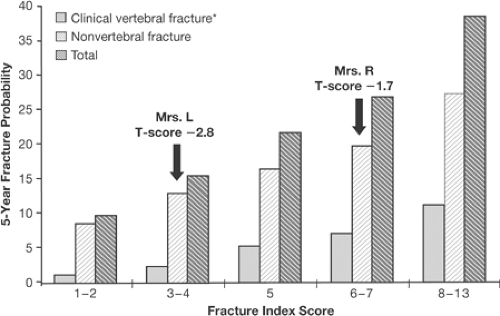
A number of papers have been published that look at the interaction of risk factors and fracture risk prediction [30,31,32]. All of these studies show that as the number of risk factors increases, the risk for hip or all fractures also increases. In addition, above a certain number of risk factors [4,5] the risk for fracture begins to plateau, and it is clear that certain risk factors have more power to predict risk than others: prior fracture, increased age, low BMD, and family history of osteoporosis are easily captured in clinical practice. The risk factor assessment also shows that a “T-score is not a T-score is not a T-score.” For example, in the 5-year fracture risk score by Black et al. [31] (Fig. 9.3), Mrs. L, with a T-score of -2.8, has a lower 5-year fracture risk than Mrs. R, with a T-score of -1.7. This is related to the fact that the fracture risk of Mrs. L is based on the risk calculated from low BMD and age alone, whereas the presence of a prior fracture and smoking in Mrs. R puts her at greater risk for fracture, even though her T-score is better. Thus, just basing risk and intervention decisions on a BMD (or T-score) level alone for the osteopenic population becomes problematic. What level of BMD or T-score should be a cutoff
for intervention? There is no clear answer, but progress in this arena has been made.
for intervention? There is no clear answer, but progress in this arena has been made.
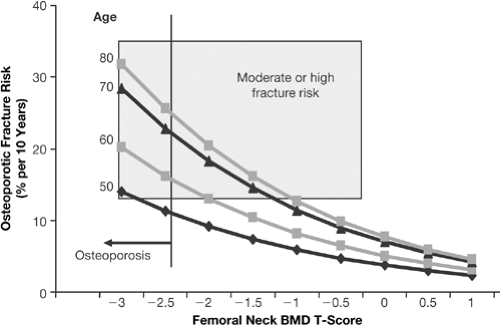
It appears that the WHO diagnostic threshold for osteoporosis of a T-score of -2.5 is close to an intervention threshold of -2.5 for treating a postmenopausal woman at age 50 years with the intent of reducing the 10-year absolute fracture risk for all (global) fractures to less than 10% [33] (Fig. 9.4). The WHO absolute fracture risk validation data have spearheaded the establishment of intervention thresholds based on 10-year longitudinal population studies representing data collection from more than 90,000 untreated postmenopausal women. The individual risk factors that have been validated as consistently predicting fracture risk are shown in Table 9.3. The 10-year risk for all fractures at age 50 years and a T-score of -2.5 is 10%. What level of risk is an unacceptable risk? This question may be answered from both a health-economic and a clinical point of view. From the WHO perspective, the treatment threshold is a health-economic one and may be decided from country to country based on the gross domestic product (GDP) of each nation and the disutility costs of the fracture(s). The treatment threshold will also vary according to the cost of the specific pharmacological therapy. For example, for the United States, the intervention threshold may be set at 10% fracture risk or greater. Hence, from the health-economic perspective, physicians and payors may provide treatment based on a risk for all fractures over 10 years of 10% or greater. This would mean at age 50 years, a postmenopausal woman with a T-score of -2.5 or lower [at the femoral neck, based on calculation from the National Health
and Nutrition Examination Survey III (NHANES III) database] would receive treatment for osteoporosis.
and Nutrition Examination Survey III (NHANES III) database] would receive treatment for osteoporosis.
The treatment threshold would be different (e.g., T-score of -1.5) if the patient had additional risk factors that, when added to low BMD and age alone, would also yield a fracture risk over 10 years of 10% or more. Hence, a postmenopausal woman at age 50 years with a family history of osteoporosis and who is also a smoker would be treated at a T-score of -1.5. Thus, the WHO absolute fracture risk validated data will allow the tailoring of treatment based on decisions that incorporate more than just the T-score level and help move the field forward to a risk-based treatment decision process.
Table 9.3. World Health Organization population-based validated risk factors for global fracture risk over a 10-year period | ||||||||
|---|---|---|---|---|---|---|---|---|
|
From a clinical point of view, the WHO absolute fracture risk model has limitations. The WHO model does not include morphometric vertebral fractures or bone turnover markers, both of which are known to independently predict fracture risk as well [34,35]. The WHO risk project did not include morphometric vertebral fractures or bone turnover markers in the risk model because these two risk factors were not captured in the 12 population studies that were used to validate the other risk factors.
In all clinical trial datasets that have led to FDA approval of all of the pharmacological therapies for PMO, the presence of a morphometric vertebral fracture is consistently associated with an increased risk for future fracture in the placebo arms. Had the WHO been able to add morphometric vertebral fractures into the risk predictive model, the 10-year risk would be greater than the risk based on T-score and age without a morphometric fracture. Since more morphometric vertebral fractures than clinical (painful) vertebral fractures occur in women and men after the age of 50 years, risk could be underestimated if morphometric vertebral fractures are missed.
How do clinicians resolve this important issue—to use the WHO absolute fracture risk model that, it is hoped, will be added to the dual-energy x-ray absorptiometry (DXA) computer printouts, yet also judge treatment if a morphometric vertebral fracture is identified?
The International Society for Clinical Densitometry (ISCD) has spearheaded the application of vertebral fracture assessment (VFA) by DXA as a respected means of identifying vertebral fractures and has developed indications for VFA performance and Medicare reimbursement [36]. The National Osteoporosis Foundation (NOF) is charged with leading the implementation of the use of the WHO absolute fracture risk model in the United States. It is probable that the NOF clinical report on the WHO risk model will also state that the identification of a morphometric vertebral fracture means that treatment should be considered, even if the WHO absolute calculated risk is not 10% or more. This is because of the high risk of future fracture (for all fractures) that is seen from the placebo arms of the clinical trials once a morphometric vertebral fracture is identified. In addition, from specific population data as well, the presence of a morphometric vertebral fracture is also associated with a greater risk for future fracture [37,38]. Hence, individual physicians will be able to tailor treatment decisions based on risk beyond just the WHO absolute model alone. This individual decision is clear if a prior morphometric vertebral fracture is identified.
Other risk factors that clinicians often use to individualize treatment are bone turnover markers (BTMs), especially markers of bone resorption. In several studies, a high rate of bone turnover is associated with a greater risk for fracture, independent of the prevailing BMD [34,35,39]. In addition, published studies indicate that the rate of postmenopausal bone loss has some correlation to the baseline level of bone turnover—for example, the higher the BTM, the greater the rate of bone loss [36]. Hence, a clinical decision may be made if the BTM is high as opposed to normal. For example, one might consider treatment in a postmenopausal woman with a T-score of -1.4 if her resorption marker is high as opposed to normal or low. Although there is no evidence that response to osteoporosis pharmacological therapy
is greater (e.g., fracture risk reduction) if the BTM is high or normal, the decision to treat may be triggered if one believes that a woman who is concerned about osteoporosis prevention has a greater rate of loss and fracture risk based on a high BTM. Much of this type of decision based on BMD and bone turnover marker levels has to do with individual patient concerns about maintaining BMD at the current level. In theory, this approach is based on maintenance of BMD, such that the BMD will not be lower 10 years later when the fracture risk could be higher due to older age. Other considerations include preserving BMD at the current level to reduce the lifetime risk of fracture, an untested consideration. Here, the concept is that if BMD continues to decline over a patient’s lifetime, the risk for fracture is greater as the patient gets older. Though the lifetime fracture risk as a function of the menopausal BMD (or T-score) is not validated, the concept is valid—based on the continual rate of bone loss seen after the menopause in untreated women, the lower the BMD will become as age increases. Accompanying a lower BMD and an older age will be a greater fracture risk. Thus, earlier intervention in younger postmenopausal women without osteoporosis by WHO criteria is often tailored to mitigating lifetime fracture risk.
is greater (e.g., fracture risk reduction) if the BTM is high or normal, the decision to treat may be triggered if one believes that a woman who is concerned about osteoporosis prevention has a greater rate of loss and fracture risk based on a high BTM. Much of this type of decision based on BMD and bone turnover marker levels has to do with individual patient concerns about maintaining BMD at the current level. In theory, this approach is based on maintenance of BMD, such that the BMD will not be lower 10 years later when the fracture risk could be higher due to older age. Other considerations include preserving BMD at the current level to reduce the lifetime risk of fracture, an untested consideration. Here, the concept is that if BMD continues to decline over a patient’s lifetime, the risk for fracture is greater as the patient gets older. Though the lifetime fracture risk as a function of the menopausal BMD (or T-score) is not validated, the concept is valid—based on the continual rate of bone loss seen after the menopause in untreated women, the lower the BMD will become as age increases. Accompanying a lower BMD and an older age will be a greater fracture risk. Thus, earlier intervention in younger postmenopausal women without osteoporosis by WHO criteria is often tailored to mitigating lifetime fracture risk.
A few other non-WHO-validated independent risk factors for fracture include a long hip axis length (HAL), hip structural analysis (HSA), and the Femur Strength Index (FSI). These parameters, which are capable of being measured by DXA and are available in specific DXA softwares, have been shown in small studies to predict hip fracture risk, independent of the hip BMD level [40,41,42]. The most recent report of these DXA-derived strength parameters is the Hip Strength Analysis Program, which incorporates the HAL, cross-sectional moment of inertia (CSMI), and the Femur Strength Index. The FSI is the ratio of the estimated compressive yield strength of the femoral neck to the expected compressive stress of a fall on the greater trochanter [43]. The FSI was significantly lower in hip-fractured patients as compared with controls, as the HAL was significantly higher than in controls, and these two strength parameters predicted hip fracture independent of the prevailing T-score. Hence, once these interesting preliminary findings are validated in larger sample sizes and we develop means of modifying these parameters, these DXA software applications will take on greater clinical meaning both for risk prediction and for additional therapeutic interventions.
The considerations for tailoring therapy to individual patients should include strategies to modify frailty. Abundant literature shows that frailty can be quantitated in the elderly population, and that certain “frailty scores” are associated with a greater risk for falling and hip fractures [44]. In addition, certain frailty parameters can be modified to reduce frailty and reduce the risk for falls [45]. For example, lower-extremity muscle tone and balance can be improved with specific interventions and are associated with a lower hip fracture risk [46].
Fall Prevention Strategies
Falls are a greater contribution to hip fracture than low BMD in the more elderly population [24]. In addition, specific pharmacological therapy that reduces hip fracture risk in specific populations may not be as effective in
the more elderly population [11]. In this latter cited clinical trial, falls were not captured in the dataset in this population, 80 years of age and older, randomized on the basis of risk factors for falling, so it remains unknown if more falls were the reason that pharmacological treatment was ineffective. Notwithstanding, it seems logical to target fall prevention strategies in frail individuals that may have a greater impact on reducing hip fractures than pharmacological therapies.
the more elderly population [11]. In this latter cited clinical trial, falls were not captured in the dataset in this population, 80 years of age and older, randomized on the basis of risk factors for falling, so it remains unknown if more falls were the reason that pharmacological treatment was ineffective. Notwithstanding, it seems logical to target fall prevention strategies in frail individuals that may have a greater impact on reducing hip fractures than pharmacological therapies.
Treatment to Reduce Risk of Specific Types of Fractures or in Patients with Specific Needs
Early Postmenopausal Women
HT is useful in preventing postmenopausal bone loss; in women who also have menopausal symptoms, it is the intervention of choice that can accomplish two end points. Whereas menopausal symptoms are the first indication for HT, the physician can also feel confident that for the majority of women who receive proper formulations of HT, their skeleton can also be protected [2]. The WHI data are very clear on this outcome. The dose of conjugated equine estrogen (0.625 mg/day) reduced both clinical vertebral as well as hip fractures. While not FDA labeled for the treatment of PMO, the lack of an FDA registration does not mean that there is a lack of evidence for a fracture reduction benefit.
The same statement can be said of many agents FDA registered for the treatment of PMO. Although the pivotal clinical trial that led to the FDA registration of raloxifene—MORE (multiple outcomes raloxifene evaluation)—had powerful vertebral fracture reduction data, no effect was demonstrated on a risk reduction for nonvertebral or hip fractures [5]. Although this lack of efficacy does not necessarily mean that raloxifene could not reduce the risk of nonvertebral or hip fractures in the context of a different clinical trial design, it does suggest that the target population for raloxifene should be postmenopausal women with a low spine BMD and normal hip BMD, or younger (50–65 years) postmenopausal women with a vertebral fracture and low risk for hip fracture.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree