This review by a 10-member panel of experts in surgical prehabilitation addresses processes that may improve oncologic care. Surgical prehabilitation is the process on the continuum of care that occurs between the time of cancer diagnosis and the beginning of surgical treatment. The panel focused on the current state-of-the-science and recommended future research that would help to identify the elements that enhance preoperative physical, nutritional, and psychological health in anticipation of surgery, mitigate the burden of disease, facilitate the return of patient health status to baseline values, decrease postoperative morbidity, and reduce health care costs.
Key points
- •
Surgical prehabilitation is the process on the continuum of care that occurs between cancer diagnosis and surgical treatment.
- •
Physiologic principles support the implementation of either unimodal or multimodal preoperative interventions in patients diagnosed with cancer and requiring surgical intervention.
- •
Recommendations are proposed to advance research in surgical prehabilitation by identifying the role of exercise, nutritional optimization, and psychological stress reduction in order to increase physiologic reserves in anticipation of surgery.
- •
There is a need to determine the impact of prehabilitation on length of stay, unanticipated readmissions and emergency department visits, perioperative complications, short-term impairments, long-term impairments, late effects, and associated disability and delays to planned postsurgical oncologic treatment.
Introduction
Surgery remains a cornerstone of oncology treatment, and minimally invasive approaches, enhanced recovery pathways (ERPs), and other interventions have improved safety and patient outcomes. However, despite these advances, major cancer resections of the bladder, pancreas, lung, or esophagus have mortalities of 4% to 9% and high morbidities persist even for lower-risk procedures like colorectal resection, ranging from 25% to 50%. Postoperative complications prolong hospital lengths of stay, increase readmissions and elevate costs, impact patient functioning and quality of life, and may have long-term implications on mortality. Tissue trauma, reduced physical activity, quasi-starvation, and psychological distress associated with major surgery result in a rapid decline in functional capacity, followed by slow recovery. At-risk populations, including the elderly, are more susceptible to the negative effects of surgical stress, and some never regain their baseline functioning. Poor preoperative fitness and physical status are risk factors for serious postoperative complications and prolonged disability. Neoadjuvant oncologic therapies may be associated with additional degradations of physical fitness before surgery.
The preoperative period may provide an opportunity to increase the physiologic reserve in the anticipation of neoadjuvant therapies and surgery with the intention to improve outcomes and accelerate recovery ( Fig. 1 ). Much as someone might train for any upcoming physical challenge, prehabilitation is a compelling strategy to address modifiable risk factors that impact cancer treatment outcomes.
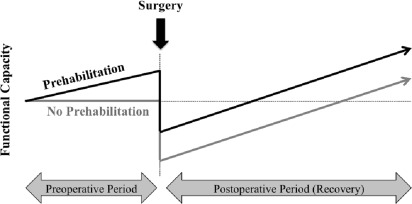
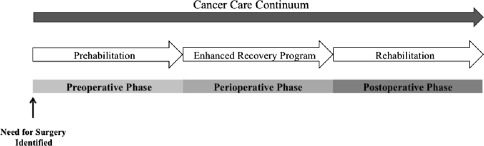
Cancer prehabilitation is “A process on the cancer continuum of care that occurs between the time of cancer diagnosis and the beginning of acute treatment and includes physical and psychological assessments that establish a baseline functional level, identify impairments, and provide interventions that promote physical and psychological health to reduce the incidence and/or severity of future impairments” ( Fig. 2 ). Of note, prehabilitation is not a “one size fits all” program before surgery, but rather involves specific individualized assessments and interventions that are likely to improve outcomes for each patient. Much of the early cancer prehabilitation literature focused on exercise as a single modality intervention ; however, recent reports have investigated other modalities such as nutritional and psychological interventions either alone or in combination with exercise. This expanding scope of prehabilitation is likely due to the acknowledgment that non–exercise interventions may be beneficial as well as that prescribing exercise as a single modality shortly before surgery may actually be detrimental to some patients who lack physiologic reserves. For example, frail elderly patients known to be at high risk for postoperative complications often present with decreased muscle mass and low protein reserves, and they may not tolerate an increase in exercise before surgery without protein supplementation. Although there is encouraging evidence in support of surgical prehabilitation in abdominal surgery, much remains to be studied.
Beginning in early 2015, a 10-member panel of Canadian and US prehabilitation subject matter experts was invited to work collaboratively in an effort to describe the current state-of-the-science in oncology surgical prehabilitation. The panel then convened in November 2015 for 2 days at the McGill University Health Centre in Montreal, Quebec, Canada, to discuss their findings and reach consensus on recommendations for future research directions. This report summarizes the current state-of-the-science and recommends directions for future research.
Introduction
Surgery remains a cornerstone of oncology treatment, and minimally invasive approaches, enhanced recovery pathways (ERPs), and other interventions have improved safety and patient outcomes. However, despite these advances, major cancer resections of the bladder, pancreas, lung, or esophagus have mortalities of 4% to 9% and high morbidities persist even for lower-risk procedures like colorectal resection, ranging from 25% to 50%. Postoperative complications prolong hospital lengths of stay, increase readmissions and elevate costs, impact patient functioning and quality of life, and may have long-term implications on mortality. Tissue trauma, reduced physical activity, quasi-starvation, and psychological distress associated with major surgery result in a rapid decline in functional capacity, followed by slow recovery. At-risk populations, including the elderly, are more susceptible to the negative effects of surgical stress, and some never regain their baseline functioning. Poor preoperative fitness and physical status are risk factors for serious postoperative complications and prolonged disability. Neoadjuvant oncologic therapies may be associated with additional degradations of physical fitness before surgery.
The preoperative period may provide an opportunity to increase the physiologic reserve in the anticipation of neoadjuvant therapies and surgery with the intention to improve outcomes and accelerate recovery ( Fig. 1 ). Much as someone might train for any upcoming physical challenge, prehabilitation is a compelling strategy to address modifiable risk factors that impact cancer treatment outcomes.
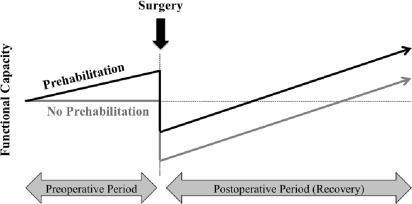
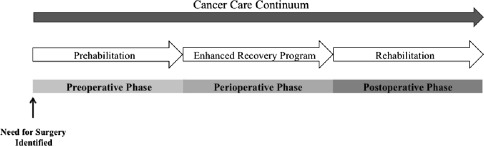
Cancer prehabilitation is “A process on the cancer continuum of care that occurs between the time of cancer diagnosis and the beginning of acute treatment and includes physical and psychological assessments that establish a baseline functional level, identify impairments, and provide interventions that promote physical and psychological health to reduce the incidence and/or severity of future impairments” ( Fig. 2 ). Of note, prehabilitation is not a “one size fits all” program before surgery, but rather involves specific individualized assessments and interventions that are likely to improve outcomes for each patient. Much of the early cancer prehabilitation literature focused on exercise as a single modality intervention ; however, recent reports have investigated other modalities such as nutritional and psychological interventions either alone or in combination with exercise. This expanding scope of prehabilitation is likely due to the acknowledgment that non–exercise interventions may be beneficial as well as that prescribing exercise as a single modality shortly before surgery may actually be detrimental to some patients who lack physiologic reserves. For example, frail elderly patients known to be at high risk for postoperative complications often present with decreased muscle mass and low protein reserves, and they may not tolerate an increase in exercise before surgery without protein supplementation. Although there is encouraging evidence in support of surgical prehabilitation in abdominal surgery, much remains to be studied.
Beginning in early 2015, a 10-member panel of Canadian and US prehabilitation subject matter experts was invited to work collaboratively in an effort to describe the current state-of-the-science in oncology surgical prehabilitation. The panel then convened in November 2015 for 2 days at the McGill University Health Centre in Montreal, Quebec, Canada, to discuss their findings and reach consensus on recommendations for future research directions. This report summarizes the current state-of-the-science and recommends directions for future research.
The role of exercise in surgical prehabilitation
Exercise in the Cancer Trajectory
Regular exercise has long been recognized as an effective means of preventing disease. Cardiac and other types of rehabilitation have also incorporated exercise into comprehensive disease management. Studies of subjects confined to bed rest highlight the rapid loss of physical function and insulin sensitivity that occur with sedentary behavior.
Robust evidence supports the role of structured exercise as a means of enhancing diverse outcomes during and following the active phase of cancer treatment. Of late, exercise is also gaining acceptance for its potential in the preintervention period. As it becomes increasingly clear that exercise plays an important role in both cancer prevention and treatment, tailoring validated exercise programs can be challenging in this patient population.
Precisely defining exercise is challenging because there are many definitions in the literature. For the purposes of this report, exercise encompasses regular physical activity that is incorporated into a planned and structured program. This planned program contrasts with generic recommendations to increase physical activity preoperatively. Furthermore, exercise designed to improve overall cardiovascular or muscular fitness is distinct from exercise that is, “targeted” and focuses on the training or retraining of specific muscles to facilitate disease-specific outcomes such as improving swallowing in head and neck cancer survivors, urinary continence in patients with prostate cancer, and reducing arm, shoulder, and upper quadrant pain and disability in patients with breast cancer.
This discussion is focused on the overall cardiovascular and strength training components of exercise with specified intensity, frequency, and modality, as more prehabilitation research currently supports this than “targeted” exercise. However, future studies may demonstrate the benefit of targeted exercises in optimizing patient outcomes.
Prescribing Exercise in the Prehabilitation Period
At the present time, there are no guidelines specific for general cardiovascular or resistance exercise in the prehabilitation period. However, the American Cancer Society has published broad exercise guidelines that recommend at least 150 minutes of moderate or 75 minutes of vigorous intensity (or combination of) exercise per week and include 2 to 3 sessions of resistance training, involving major muscle groups. An individual’s baseline health and fitness, as well as cancer diagnosis, treatment sequelae, and comorbid conditions, should inform exercise prescribing for the patient with cancer. A growing body of evidence highlights the need to consider the type and amount of activity that is performed during non-exercise time. Adequate recovery/rest time (both between sets and sessions) should be included in the prescription, especially if the individual is unaccustomed to the prescribed activity. When considering exercise as a prehabilitation intervention, “one size does not fit all.” Exercise prescriptions are ideally tailored to baseline levels of fitness (determined by formal assessment), current health status, and the planned surgical procedure. In some individuals, exercise is contraindicated altogether or should be modified based on their health status, and safety in the cancer population is an important consideration.
There is a need in patients with cancer to compare the cost-effectiveness, health benefits, and adherence rates of different modes of exercise delivery (eg, supervised personal training, supervised group training, home-based training). It is also important to establish who should be supervising these exercise programs and what qualifications they should have in order to ensure the safety and address the specific needs of a patient. Sensor technology has only recently allowed for the generation of objective data to inform the discussion of fatigue and health-related quality of life (HRQOL), as well as to inform care delivery. In oncology, the main focus of sensor-based analyses has been in the survivor population. Trials using sensor technologies have shown a relationship between moderate to vigorous physical activity (MVPA), sedentary time (SED), and health-related quality of life (HRQOL). Examples include a study of 199 breast cancer survivors showing that levels of pain, fatigue, and dysphoria are related to the amount of MVPA and SED experienced, and a study showing that participants who achieved 150 minutes of MVPA per week had 18% higher HRQOL relative to those who reported no MVPA. Even more nascent, yet equally important, is the use of sensors in the analysis of sleep among oncology patients. The rate of sleep disturbance among patients with cancer ranges from 25% to 59%, and the quality of sleep may impact quality of life. The increasing use of sensors in the community and acceptance by the public have paved the way to integrating them into research studies and clinical care.
The role of nutrition in surgical prehabilitation
Malnutrition and Nutritional Risk
Malnutrition arises from inadequate intake and/or metabolic and inflammatory alterations that alter nutrient requirements or absorption, which, ultimately, leads to wasting and diminished physical function. The cause of malnutrition is generally multifactorial and includes gastrointestinal (GI) abnormalities (eg, nausea, diarrhea), tumor-related mechanisms (eg, obstruction), and tumor-induced metabolic abnormalities (eg, insulin resistance, catabolism), as well as anticancer therapies that provoke anorexia and GI derangements. Data on the prevalence of malnutrition vary based on tumor type, site, and advancement of disease as well as anticancer treatment; however, the prevalence of malnutrition associated with body wasting among patients with cancer with either early or late disease has been estimated to range between 28% and 57%. Perioperative treatment of disease-related malnutrition with oral nutrition supplements or enteral nutrition might reduce rates of mortality and morbidity. Moreover, recent North American surgical consensus recommendations suggest moving beyond treating malnutrition to proactive preoperative nutritional therapy in all “at-risk” patients to potentially mitigate complication rates and severity. As a result, early determination of malnutrition risk, for the purpose of eliciting a comprehensive dietary consult, throughout the continuum of care for oncologic and surgical patients, is increasingly recognized as a significant component of quality care.
A systematic approach to identifying and treating patients at nutritional risk should be established. Several nutrition screening tools, such as the Nutrition Risk Screening 2002 and Subjective Global Assessment, have been used and validated in surgical populations.
Nutritional Care Goals
The primary goal of oncologic and perioperative nutritional care is to decrease the incidence and severity of nausea and vomiting, improve appetite recovery, enhance immunity, support normoglycemia, and provide sufficient protein intake to achieve anabolism and enough energy to maintain body weight. It is becoming increasingly evident that these interventions should begin preoperatively . The use of appropriate assessments, such as that to detect malnutrition, before surgery permits patient-specific treatments that can improve metabolic status. The goals would include improved postoperative outcomes as well as less nutritional support needed after surgery.
Optimal Nutrition Care: A Combination of Oral Nutrition Supplements and Dietary Counseling
A Cochrane Review did not find a reduction of complications or length of hospital stay with the use of standard preoperative oral nutritional supplements in patients undergoing GI surgery. Although some benefits were found with immune-enhancing nutrients, these results cannot yet be generalized to the ERP population. A combination of both individualized nutrition counseling and oral nutritional supplements has been proven to be effective in building functional capacity in prehabilitation trials.
Integrating Nutrition with Exercise
Observational evidence suggests that patients with higher preoperative lean body mass (ie, reserve) are better able to cope with surgical stress as determined by reduced complications and earlier discharge. In order to generate a positive net protein balance in favor of lean body mass accretion, exogenous amino acids must be administered to produce a state in which protein synthesis exceeds that of protein breakdown. Twenty grams of protein (in liquid form) taken immediately after resistance exercise is regarded as sufficient to maximally stimulate muscle protein synthesis in healthy individuals. The optimal after-exercise diet to support lean body mass accretion in patients with cancer should be investigated. Although most of the literature has focused on assessing malnutrition and providing interventions to those with documented problems, a recent study suggests that even patients with cancer with no clinical signs of malnutrition may have better outcomes with dietary intervention. Finally, omega-3 fatty acids, particularly eicosapentaenoic acid and docosahexaenoic acid, which are found naturally in fish oils, have been identified in several randomized controlled trials to reduce oxidative stress and inflammation in cancer and surgical patients, which may translate into minimized loss of lean body tissue. Adequate dietary intake of these nutrients should thus be a consideration in dietary planning.
The role of psychological stress reduction in surgical prehabilitation
Emotional Stress and Its Impact on Perioperative Outcome
A recently published systematic review on psychological surgical prehabilitation suggests a positive role. A large body of published literature suggests associations of patients’ preoperative psychological state with their postoperative recovery, including wound healing, infection, function, and length of hospital stay. For instance, anxiety and depression can predict surgical outcome, even after known physiologic factors are accounted for. Presence of depression increases the length of hospitalization for patients undergoing thoracic surgery for cancer and is associated with poor compliance to medical treatment.
Preoperative anxiety and its concomitant stress response are the most frequently cited psychological factors to affect wound healing, hospital stay, return to function, and satisfaction with surgery. Trait and state anxiety have been shown to have significant effect on postoperative complications and impaired wound healing. The diagnosis of cancer and the addition of neoadjuvant chemoradiation therapy enhance even further the emotional stress in anticipation of surgery.
Preoperative Strategies to Attenuate Anxiety
Evidence-based perioperative psychological interventions effectively reduce anxiety in surgical patients. Reduced anxiety has been empirically linked to improvements in patients’ immunologic function, as well as self-reported psychological outcomes, quality of life, and somatic symptoms. However, these strategies did not affect traditional surgical outcomes, including length of hospital stay, complications, analgesic use, or mortality. A wide variety of anxiety-reducing approaches have been used, including relaxation training and education, deep breathing exercises, visualization, yoga, and music in the perioperative setting. Music therapy can reduce postoperative anxiety, pain, and analgesia requirements as well as improve patient satisfaction, by reducing sympathetic arousal.
The role of smoking cessation in surgical prehabilitation
The literature shows that patients with cancer who successfully abstain from smoking before surgery have reduced risk of postoperative complications and improved performance status and quality-of-life measures. In contrast, patients with cancer who continue to smoke face an increased risk of postoperative complications, impaired wound healing, delays to adjuvant chemotherapy, increased recurrence and second primaries, and increased mortality.
Although smoking cessation has not been well studied as a prehabilitation intervention, this panel of subject matter experts recommends addressing nicotine dependence at the time of cancer diagnosis, initiating smoking cessation therapy, and using the eventual hospitalization to reinforce abstinence. Best practices in smoking cessation suggest counseling along with either combination nicotine replacement therapy (cNRT) or Varenicline. cNRT is a recommended first-line treatment especially in those more motivated to quit. Prescriptions consist of appropriately dosed transdermal nicotine (patch) and a short-acting nicotine to be used as needed for cravings. If there are no contraindications, Varenicline is an equally good choice and may be a preferred treatment in the ambivalent smoker due to its flexible target quit date of 8 to 35 days. Nicotine replacement can be combined with Varenicline to help additional cravings. Either NRT or Varenicline monotherapy may also be used in those patients who are not ready to quit but are willing to reduce tobacco consumption ( Table 1 ).








