Surface Replacement Arthroplasty of the Shoulder
David S. Bailie
Todd S. Ellenbecker
INDICATIONS AND CONTRAINDICATIONS
Primary and secondary arthritis of the shoulder are the common indications. The prosthesis has been used for osteoarthritis (OA), rheumatoid arthritis, avascular necrosis (AVN), cuff arthropathy, instability arthropathy, post-traumatic arthritis, postinfectious arthritis, arthritis secondary to glenoid dysplasia, and epiphyseal dysplasia. It is not intended for use in fresh fractures.
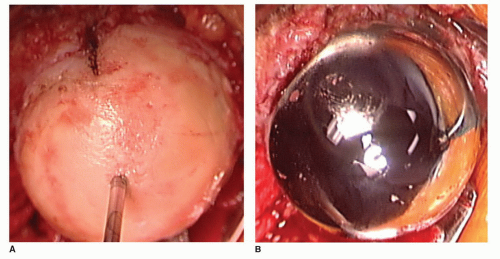
While resurfacing arthroplasty of the shoulder is a concept that has been utilized since the 1970s, it was in 1979 that this implant, the Copeland design, was initially designed by Stephen A. Copeland, FRCS (Br), at the Reading Shoulder Unit in Reading, United Kingdom. It was first implanted in 1986. Early designs of this device (i.e., Copeland implant) included a cobalt chromium alloy implant with a central peg and screw. It was initially used in conjunction with a glenoid component. Several modifications were made resulting in the current version of a cobalt chromium shell with a central peg and hydroxyapatite coating, suitable for noncemented applications (Fig. 39-1).
The ideal humeral replacement implant should be designed so as to reproduce normal anatomy (1). This includes variable humeral version of 5-degree anteversion to 55-degree retroversion and variable radius of curvature. An ideal implant should preserve bone stock, restore lateral offset, be flexible and adaptable intraoperatively, be revisable, and be durable (including bearing surface and method of fixation). Altering offset, inclination, or version can alter shoulder kinematics. Given the variations, implants must allow for adaptability during surgery. These criteria for any shoulder arthroplasty are embodied in the most widely used resurfacing implant, that designed by Dr. Copeland.
The rationale for using a resurfacing implant over one of the stemmed designs, include (a) true anatomic head coverage (not replacement), (b) bone preservation, (c) easy revisability to total shoulder arthroplasty (TSA), and (d) easy conversion to arthrodesis, in rare cases. Retention of the native head and neck of the humerus will preserve the biomechanics of the joint. This technique also eliminates the risk of humeral fracture or perforation during implantation and allows the head position to be independent of the shaft. The latter is important in cases where proximal humerus malunions or other deformity may preclude access to the humeral shaft. As the shaft is not violated, it minimizes the risk of periprosthetic fractures postoperatively. The design inherently allows placement in infinite variations of version, offset, and angulation. Since no humeral canal reaming or cementing is required, it eliminates the risk of fat embolus or hypotension during humeral preparation and implantation.
In cases of prior elbow arthroplasty, intramedullary nailing of the humerus and prior plating of the humerus resurfacing allow for anatomic arthroplasty without having to remove all of the hardware. If there has been a proximal humeral malunion, this can be left undisturbed without worry about the head offset relative to the shaft.
Specific indications for resurfacing arthroplasty vary among authors. Copeland and colleagues prefer this implant for all cases of primary shoulder arthroplasty where a stemmed implant would be considered, except fresh fractures (2). For primary arthroplasty, we have focused on the use of resurfacing in the younger or more active patient where a stemmed implant may force an alteration in lifestyle as a result of implant failure concerns.
Although we have not directly compared the resurfacing implants and standard stemmed hemiarthroplasty or TSA in active patients, we have observed a much greater return to sports and active lifestyle in the resurfacing population and have not observed implant failure as a result. This includes in several of our cases athletes who participate in collision sports.
Although we have not directly compared the resurfacing implants and standard stemmed hemiarthroplasty or TSA in active patients, we have observed a much greater return to sports and active lifestyle in the resurfacing population and have not observed implant failure as a result. This includes in several of our cases athletes who participate in collision sports.
Currently, our choice in primary arthroplasty is to reserve resurfacing arthroplasty for active individuals (generally younger than 60 years of age, although age alone is not an indicator) who do not have contraindications; we continue to utilize stemmed TSA, when possible, for all others (3).
There are few absolute contraindications to a resurfacing arthroplasty. As with any other partial or total shoulder replacement, active infection or paralysis of both deltoid and rotator cuff muscles are contraindications. Additional contraindications, in our practice, include (a) significant subchondral cystic changes on either side of the joint as can be seen with advanced rheumatoid arthritis (RA) or postarthroscopic glenohumeral chondrolysis (PAGCL) from prolonged intra-articular infusion of local anesthetics, (b) acute fracture, (c) uncontained humeral head defects that may compromise component fixation, (d) soft humeral head bone that may collapse under the implant (may be determined at time of surgery), (e) contained AV N involving more than 40% of the humeral head, (f) tumors, and (g) significant glenoid erosion requiring a glenoid replacement. The latter situation could be addressed as a resurfacing TSA, but we find it simpler and more predictable to do a standard stemmed TSA in those cases. However, there have been rare cases where we have used a biologic resurfacing technique for the glenoid. This has included (a) cases of lateral meniscus allograft when there is posterior subluxation from an early biconcave glenoid in a highly active individual and (b) the use of dermal allograft covering of contained defects of the glenoid that we have filled with bone graft, as frequently seen in failed Bristow procedures. Because biologic glenoid resurfacing tends to be less predictable, we have started to be less aggressive on the glenoid side if we have chosen a hemiarthroplasty instead of a TSA. More often, the glenoid is either left alone or only the remaining articular cartilage (in the case of 50% loss or more) is removed without altering the subchondral plate and version of the glenoid face. As stated previously, resurfacing is done in primary arthroplasty in approximately one-third of our cases.
PREOPERATIVE PLANNING
Cuff Tear Arthropathy
Patients with massive nonrepairable rotator cuff tears with arthropathy (CTA) are often in need of surgical management to alleviate pain. In our clinic, we have identified two distinct groups of patients with this disorder. Functional CTA (FCTA) include those with greater than 90 degrees of elevation with an insidious onset of pain. Older patients, who may have had multiple prior rotator cuff surgeries or injections, often have nonfunctional CTA (NFCTA) with anterior-superior escape and elevation less than 90 degrees. If surgery is contemplated in the latter, reverse arthroplasty is the procedure of choice.
FCTA patients have pain and weakness but preserved function (often elevation greater than 150 degrees). Their shoulder has adapted to the loss of the rotator cuff and the deltoid has the power to initiate and maintain abduction and elevation. If conservative treatment in this group fails (i.e., no more than two annual corticosteroid injections and deltoid retraining rehabilitation), then arthroplasty can be considered to alleviate pain and preserve function. Our preference is a resurfacing head with an extended articular surface (Copeland EAS, Biomet Inc, Warsaw, IN). The benefit of this implant is that the structures that are allowing for compensated shoulder function are not disturbed. By preserving the humeral head and replacing only the diseased cartilage surface, pain is relieved (Fig. 39-2). At the same time, the thickness of the implant allows for some restoration of the center of rotation to a more lateral and inferior position, thus improving deltoid power (Fig. 39-3).
Results have been encouraging with adequate pain relief and preservation of function, although we have not seen predictable strength gains. In the event of decompensation to an NFCTA, the EAS can easily be removed and revised to a reverse arthroplasty, when indicated.
Results have been encouraging with adequate pain relief and preservation of function, although we have not seen predictable strength gains. In the event of decompensation to an NFCTA, the EAS can easily be removed and revised to a reverse arthroplasty, when indicated.
SURGERY
The patient is given an interscalene block while awake using nerve stimulator control. The surgeon marks the operative site and antibiotic prophylaxis is given within 1 hour of incision. The patient is positioned on a table adapter to allow for free arm motion, including full rotation and extension, while maintaining head stability. The table is inclined to elevate the head approximately 30 degrees. A time-out is called prior to starting the surgery and all implants are verified.
Our surgical approach is the same as we use for standard TSA. Although the resurfacing technique is straight-forward, it requires good soft-tissue releases since the humeral head is not being removed. The humeral head must be seen en face in resurfacing and we cannot emphasize enough the importance of soft-tissue contracture releases.
While Copeland and colleagues prefer the anterosuperior approach in order to perform acromioplasty and distal clavicle excision at the same time, we use a deltopectoral approach for all arthroplasty surgery. Once the cephalic vein has been located, it can be moved medially or laterally, but we do try and preserve this structure to avoid compromise of venous outflow. The subacromial space and subdeltoid space are gently freed of adhesions. The conjoined tendon is gently retracted medially using handheld retractors. Self-retaining retractors can be used but tend to increase soft-tissue tension and limit surgical access in some cases. In addition, the musculocutaneous nerve is at risk of compression injury with a medial-based retractor. In resurfacing, the anterior circumflex vessels are preserved to minimize any risk of vascular compromise to the humeral head. The long head of biceps (LHB) is routinely tenodesed at the level of the pectoralis major tendon. This is done for several reasons: (a) to enhance exposure, (b) to avoid painful LHB adhesions after removal of loose bodies from the bicipital groove, and (c) to decrease the likelihood of post-op pain as a result of LHB tenosynovitis as activity levels increase.
The subscapularis (SSC) is released 1 cm lateral to the myotendinous junction from the rotator interval to the anterior circumflex vessels. A horizontal incision is made high in the interval and directed toward the base of the coracoid, thus releasing the superior glenohumeral ligament and coracohumeral ligament. The inferior muscular portion of the SSC is separated from the underlying capsule, the latter of which is then incised to the glenoid rim while protecting the axillary nerve with a blunt retractor or gloved finger. The anterior capsule is then left on the posterior aspect of the SSC (this helps to reinforce repair strength at closure) but incised from inferior to superior at the capsulolabral junction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree