Abstract
Background
People with hereditary and spontaneous spastic paraparesis (HSSP) report that their legs are stiffer and walking is slower when their legs are cold.
Objectives
This study explored the effects of prolonged superficial cooling and warming of the lower leg on walking speed and local measures of neuromuscular impairments.
Methods
This was a randomised pre- and post-intervention study of 22 HSSP participants and 19 matched healthy controls. On 2 separate occasions, one lower leg was cooled or warmed. Measurements included walking speed and measures of lower limb impairment: ankle movement, passive muscle stiffness, spasticity (stretch reflex size), amplitude and rate of force generation in dorsi- and plantarflexors and central and peripheral nerve conduction time/velocity.
Results
For both participants and controls, cooling decreased walking speed, especially for HSSP participants. For both groups, cooling decreased the dorsiflexor rate and amplitude of force generation and peripheral nerve conduction velocity and increased spasticity. Warming increased dorsiflexor rate of force generation and nerve conduction velocity and decreased spasticity.
Conclusions
Superficial cooling significantly reduced walking speed for people with HSSP. Temperature changes were associated with changes in neuromuscular impairments for both people with spastic paraparesis and controls. This study does not support the use of localised cooling in rehabilitation for people with spastic paraparesis as reported in other neurological conditions. Rehabilitation interventions that help prevent heat loss (insulation) or improve limb temperature via passive or active means, particularly when the legs and/or environment are cool, may benefit people with spastic paraparesis.
1
Introduction
Hereditary and spontaneous spastic paraparesis (HSSP) is a progressive condition resulting in impaired balance and walking . In type I or uncomplicated HSSP, people present lower limb paresis and spasticity because of axonal degeneration of central descending and ascending tracts including the corticospinal tract, spinocerebellar tracts and dorsal columns. In type II or complicated HSSP, additional signs include peripheral neuropathy, cerebellar ataxia or dementia . Focus groups with people with HSSP in the United Kingdom ( n = 36) highlighted the perception that their walking is often slower when their legs are cold, such as in cold weather; this is associated with an increase in perceived lower limb stiffness. Warming the lower legs by increasing layers of clothes or being in warmer environments is perceived to help with walking faster and relieve increased leg stiffness.
People with stroke or acquired brain injury show decreased spasticity, measured clinically and electrophysiologically, with periods of superficial cooling . Clinically localised cooling or cryotherapy to reduce spasticity is proposed for a range of neurological conditions . Conversely, reduced spasticity has been reported with localised and global warming . Despite the reduced spasticity, improvements in voluntary movements and function have not been clearly demonstrated , which may reflect the associated impact of temperature changes on nerve conduction velocity, passive stiffness and muscle strength.
The subjective report of improved function with warming in people with HSSP contrasts with those with multiple sclerosis, which can also be associated with upper motor-neuron syndrome. People with multiple sclerosis often report a worsening of symptoms with warming and improvement with whole body or localised cooling. This situation is mainly considered to be mediated by inducing a central nerve conduction block with warming (Uthoffs phenomenon) secondary to demyelination . Therefore, central conduction time should be assessed in HSSP.
We investigated whether:
- •
people with HSSP experience changes in walking speed and measures of neuromuscular impairments (movement, stiffness, strength and nerve conduction velocity) with prolonged superficial cooling and warming;
- •
whether these changes are comparable to that seen in healthy people. Ultimately, we aimed to determine whether rehabilitation strategies should consider the functional impact of temperature changes in people with HSSP.
2
Materials and methods
2.1
Participants
We included 22 HSSP participants and 19 healthy controls matched on age, gender and body mass index (BMI) ( Table 1 ). HSSP participants were recruited by advertisements in the UK SP support group newsletter and controls by local advertisements. HSSP participants were included if they had a diagnosis of spastic paraparesis with or without a family history. Those with other differential diagnoses were excluded by appropriate imaging, clinical and laboratory tests. Participants had to be able to walk at least 20 m with or without a walking aid and have bilateral spasticity in the ankle plantarflexors (at least grade 1 Ashworth score ). We excluded HSSP participants if they had additional orthopaedic or neurological impairments. Exclusion factors for both groups included contraindications to transcranial magnetic stimulation (TMS), poor skin integrity, Raynaud’s disease or a fixed ankle inversion contracture. Ethical approval for the study was provided by South West Cornwall and Plymouth Ethics Committee (HS13/14-105). Informed consent was provided by all participants.
| Participant | Age/gender | Family history (genetic diagnosis) | Symptom duration (year) | Barthel Index | Abbr Mental Test Score | Peripheral neuropathy | Ashworth Ankle Plantar flexors (most affected side) | Anti-spasticity medication | Additional signs and symptoms | Pure or complicated |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64F | No | 24 | 85 | 10 | No | 1 | No | No | Pure |
| 2 | 19F | Yes (SPG4) | 17 | 95 | 8 | No | 1 | Oral baclofen | No | Pure |
| 3 | 81M | Yes | 41 | 25 | 9 | Yes | 3 | Diazepam | Cerebellar ataxia, blurred vision | Complicated |
| 4 | 35F | No (SPG4) | 95 | 9 | No | 1 | No | No | Pure | |
| 5 | 68M | No | 37 | 85 | 9 | Yes | 1 | No | No | Complicated |
| 6 | 67M | Yes | 62 | 90 | 10 | No | 2 | No | No | Pure |
| 7 | 55F | No | 9 | 95 | No | 1 | Oral baclofen | No | Pure | |
| 8 | 55M | No | 11 | 50 | 9 | Yes | 1 | Oral baclofen | No | Complicated |
| 9 | 55F | Yes | 5 | 95 | 10 | No | 1 | No | No | Pure |
| 10 | 54M | Yes | 10 | 65 | 7 | Yes | 2 | Tizanadine | No | Pure |
| 11 | 69M | Yes | 6 | 100 | 10 | No | 1 | No | No | Pure |
| 12 | 63F | Yes (SPG10) | 12 | 90 | 10 | No | 1 | No | No | Pure |
| 13 | 64M | Yes | 14 | 95 | 9 | No | 2 | Botulinum toxin to plantarflexors | No | Pure |
| 14 | 65M | Yes | 15 | 90 | 9 | No | 3 | No | No | Pure |
| 15 | 48F | Yes (SPG4) | 11 | 60 | 6 | No | 3 | No | No | Pure |
| 16 | 34M | Yes (SPG4) | 2 | 100 | 10 | No | 1 | No | No | Pure |
| 17 | 54F | Yes (SPG3a) | 51 | 95 | 6 | No | 1 | No | No | Pure |
| 18 | 48M | Yes (SPG4) | 12 | 95 | 9 | No | 2 | No | Retinopathy | Complicated |
| 19 | 50F | Yes | 19 | 95 | 10 | No | 2 | No | No | Pure |
| 20 | 60M | Yes | 18 | 90 | 10 | No | 1 | No | Retinopathy | Complicated |
| 21 | 46F | No | 11 | 100 | 9 | No | 2 | Oral baclofen | No | Pure |
| 22 | 56F | Yes (SPG4) | 10 | 95 | 10 | No | 1 | No | No | Pure |
| Control | 48.2 ± 10.4 | NA | NA | 100 (0) | 10 (0) | NA | 0 (0) | NA | NA | NA |
Participants’ baseline characteristics (height, weight, age, sex, family history, genetic diagnosis, length of symptoms and presence of anti-spasticity medication) were recorded. The Abbreviated Mental Test Score was used to screen for dementia and the self-reported Barthel Index was used for functional ability. Skin-fold thickness overlying the ankle plantar flexors was measured by using a Harpenden calliper at the level of the mid-shank with the participant in a seated position and BMI was calculated from height and weight. The Ashworth scale was used to evaluate spasticity in the lower leg. HSSP was classified as pure or complicated by genetic diagnosis and the presence or absence of additional signs and symptoms, including peripheral neuropathy .
2.2
Intervention
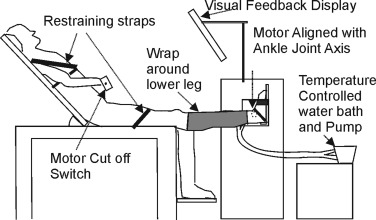
For HSSP participants, the self-reported most affected side was studied, and for healthy controls a similar proportion of dominant and non-dominant legs was assessed. Participants were assessed in a semi-reclined standardised position ( Fig. 1 ). One lower leg was cooled or warmed for 30 min by using a wrap attached to a temperature-controlled water bath with water circulating at 7 °C or 37 °C ( Fig. 1 ). The order of cooling or warming was randomised by using a computer generated code, and each condition was separated by a minimum of 24 h.
2.3
Measures
Core temperature was measured in the inner ear (tympanic membrane temperature; Omron MC 510-E2, The Netherlands). Room and shank skin temperature were measured by using thermocouples (type-t thermocouples; BAT-10 Physitemp, USA).
The primary outcome measure was maximal walking speed measured over a 10-m walkway. Two walks were recorded with a 1-min seated rest period, and the mean walking speed was calculated. Secondary outcomes were neuromuscular impairments in the lower leg. Localised movement at the ankle was measured by foot tapping time. The time taken to tap each foot 10 times was recorded with the participant in a standardised seated position. The mean foot tap time was calculated for each side.
Slow and fast stretches were used to quantify passive stiffness and stretch reflex size. A 15°-amplitude, slow (peak velocity 5 °/s) and fast (peak velocity 170 °/s) ramp stretch was applied at the ankle while the participant was relaxed. The ankle axis was aligned to the axis of a customised servomotor [Baldor BSM, UK ( Fig. 1 )]. Each stretch was repeated 6 times with a 3- to 5-s random inter-stretch interval. Torque, position (TLSF transducer, Industrial Measurements, UK) and surface electromyography (EMG) from the tibialis anterior, medial gastrocnemius and soleus muscles (2.5-cm inter-electrode distance, Digitimer D360, UK) were recorded. During the 6 slow stretches, trials were omitted if the EMG measurement was greater than the mean ± 2 SD of the pre-stretch relaxed level (baseline level). Torque, position and EMG were digitized (2 kHz Power 1401, CED Electronics, UK). EMG signals were filtered (30 Hz low-pass, 2nd-order Butterworth filtered) and rectified [MATLAB (Mathworks, USA)]. Torque and position were measured over a 300-ms period before stretch onset and immediately following stretch offset. Slow stretches were used to evaluate passive stiffness . Stiffness was normalised to body weight and defined as: ΔTorque/ΔPosition. Stretch reflex activity was characterised by the mean rectified gastrocnemius EMG above baseline following the fast stretch and used as a measure of spasticity.
Maximal isometric muscle strength (maximal voluntary contraction [MVC]) of ankle plantar- and dorsiflexors was measured using the servomotor with the ankle in 5° plantarflexion. The participant was asked to push down or pull up as hard and fast as they could and verbal encouragement was provided. The rate of torque development (MVCdt) was defined as the rate torque developed between 25–50% of the maximal torque as calculated using a least squares algorithm.
Peripheral nerve conduction was measured in the tibial nerve. The latency of abductor hallucis M waves following proximal stimulation at the level of the popliteal fossa and distal stimulation at the level of the medial malleolus were recorded. The stimulation points were marked for recording after cooling/warming and the distance between distal and proximal points were measured. Conduction velocity (m/s) was defined as inter-stimulus distance/(proximal-distal M wave latency) .
For central conduction times, motor evoked potentials (MEPs) in the abductor hallucis in response to single-pulse TMS were measured (double-cone coil, 110 mm Magstim 200 stimulator, Magstim Co., UK). Resting threshold was determined as the stimulus that produced an MEP > 50 μV in at least 3 of 5 occasions . MEP latency was measured with 3 stimuli at 1.5 × resting motor threshold up to 100% machine output (2.0 T). In 2 HSSP participants, MEPs at a resting threshold could not be determined, so MEPs were recorded at 100% machine output as they contracted the abductor hallucis muscle (∼ 10% maximal voluntary contraction). Lumbosacral roots were stimulated by using a figure-of-eight coil (70 mm) that was placed lateral to the L5 spinous process, oriented 45° to the vertical, with the coil current running in a mediolateral direction. Stimulator intensity > 80% was used to record abductor hallucis MEPs . The central conduction time was defined as motor cortex MEP latency–spinal root MEP latency.
All measures were repeated before and immediately after 30 min of superficial cooling or warming.
2.4
Statistical analysis
Tests of normality (Shapiro-Wilks) established that data from all measures were normally distributed. Baseline characteristics were compared by unpaired t -test. Changes in walking speed and neuromuscular measures of impairment were assessed by between-groups repeated-measures ANOVA, with factors being group (HSSP vs. controls), time (pre- vs. post-intervention) and temperature (cool vs. warm). An additional factor of side (targeted vs. non-targeted) was included when assessing changes in foot tap time. Results were significant at P < 0.05.
2
Materials and methods
2.1
Participants
We included 22 HSSP participants and 19 healthy controls matched on age, gender and body mass index (BMI) ( Table 1 ). HSSP participants were recruited by advertisements in the UK SP support group newsletter and controls by local advertisements. HSSP participants were included if they had a diagnosis of spastic paraparesis with or without a family history. Those with other differential diagnoses were excluded by appropriate imaging, clinical and laboratory tests. Participants had to be able to walk at least 20 m with or without a walking aid and have bilateral spasticity in the ankle plantarflexors (at least grade 1 Ashworth score ). We excluded HSSP participants if they had additional orthopaedic or neurological impairments. Exclusion factors for both groups included contraindications to transcranial magnetic stimulation (TMS), poor skin integrity, Raynaud’s disease or a fixed ankle inversion contracture. Ethical approval for the study was provided by South West Cornwall and Plymouth Ethics Committee (HS13/14-105). Informed consent was provided by all participants.
| Participant | Age/gender | Family history (genetic diagnosis) | Symptom duration (year) | Barthel Index | Abbr Mental Test Score | Peripheral neuropathy | Ashworth Ankle Plantar flexors (most affected side) | Anti-spasticity medication | Additional signs and symptoms | Pure or complicated |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 64F | No | 24 | 85 | 10 | No | 1 | No | No | Pure |
| 2 | 19F | Yes (SPG4) | 17 | 95 | 8 | No | 1 | Oral baclofen | No | Pure |
| 3 | 81M | Yes | 41 | 25 | 9 | Yes | 3 | Diazepam | Cerebellar ataxia, blurred vision | Complicated |
| 4 | 35F | No (SPG4) | 95 | 9 | No | 1 | No | No | Pure | |
| 5 | 68M | No | 37 | 85 | 9 | Yes | 1 | No | No | Complicated |
| 6 | 67M | Yes | 62 | 90 | 10 | No | 2 | No | No | Pure |
| 7 | 55F | No | 9 | 95 | No | 1 | Oral baclofen | No | Pure | |
| 8 | 55M | No | 11 | 50 | 9 | Yes | 1 | Oral baclofen | No | Complicated |
| 9 | 55F | Yes | 5 | 95 | 10 | No | 1 | No | No | Pure |
| 10 | 54M | Yes | 10 | 65 | 7 | Yes | 2 | Tizanadine | No | Pure |
| 11 | 69M | Yes | 6 | 100 | 10 | No | 1 | No | No | Pure |
| 12 | 63F | Yes (SPG10) | 12 | 90 | 10 | No | 1 | No | No | Pure |
| 13 | 64M | Yes | 14 | 95 | 9 | No | 2 | Botulinum toxin to plantarflexors | No | Pure |
| 14 | 65M | Yes | 15 | 90 | 9 | No | 3 | No | No | Pure |
| 15 | 48F | Yes (SPG4) | 11 | 60 | 6 | No | 3 | No | No | Pure |
| 16 | 34M | Yes (SPG4) | 2 | 100 | 10 | No | 1 | No | No | Pure |
| 17 | 54F | Yes (SPG3a) | 51 | 95 | 6 | No | 1 | No | No | Pure |
| 18 | 48M | Yes (SPG4) | 12 | 95 | 9 | No | 2 | No | Retinopathy | Complicated |
| 19 | 50F | Yes | 19 | 95 | 10 | No | 2 | No | No | Pure |
| 20 | 60M | Yes | 18 | 90 | 10 | No | 1 | No | Retinopathy | Complicated |
| 21 | 46F | No | 11 | 100 | 9 | No | 2 | Oral baclofen | No | Pure |
| 22 | 56F | Yes (SPG4) | 10 | 95 | 10 | No | 1 | No | No | Pure |
| Control | 48.2 ± 10.4 | NA | NA | 100 (0) | 10 (0) | NA | 0 (0) | NA | NA | NA |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





