Revision Shoulder Arthroplasty
Raymond M. Carroll
Louis U. Bigliani
INTRODUCTION
Revision of a failed shoulder arthroplasty is considered the most difficult of operative shoulder procedures (1,2). In 1982 Neer and Kirby reported the results of 40 patients with revision shoulder arthroplasty (1). On the basis of their review, two important observations were made. First, more than one factor leading to failure was present in almost every case. Additionally, the results of the majority of revision arthroplasties are inferior to those of primary arthroplasties. The lessons of this early experience with revision shoulder arthroplasty are as pertinent today as they were more than a quarter century ago.
The complexity of revision shoulder arthroplasty is not limited to the technical expertise required to perform the surgery, although it is demanding. In fact, the bulk of the article by Neer and Kirby was devoted to analyzing the cause of failure. They developed a comprehensive list of factors that individually or in various combinations might lead to failure of a shoulder arthroplasty. These factors were grouped into preoperative considerations, surgical considerations, and postoperative considerations. Neer later modified the groups (3). General causes of failure include psychological problems, nerve damage, adjacent joint arthropathy, and scapulothoracic dysfunction. Local
causes of failure include infection, instability, heterotopic bone, deltoid defects, rotator cuff defects, bone defects, contractures, and adhesions. Finally, both the humeral and glenoid implants may be a cause of failure. The majority of these factors have since been documented to contribute to the failure of a shoulder arthroplasty (4, 5, 6, 7, 8). More often than not failed shoulder arthroplasty is multifactorial (1,5). Ultimately, the treating surgeon must have a good understanding of the etiology of failure prior to pursing a surgical remedy.
causes of failure include infection, instability, heterotopic bone, deltoid defects, rotator cuff defects, bone defects, contractures, and adhesions. Finally, both the humeral and glenoid implants may be a cause of failure. The majority of these factors have since been documented to contribute to the failure of a shoulder arthroplasty (4, 5, 6, 7, 8). More often than not failed shoulder arthroplasty is multifactorial (1,5). Ultimately, the treating surgeon must have a good understanding of the etiology of failure prior to pursing a surgical remedy.
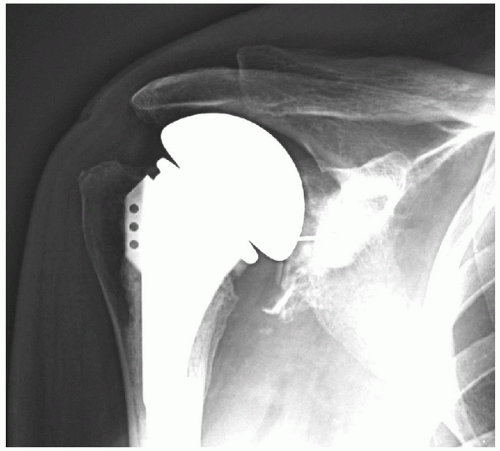
The overall complication rate for unconstrained total shoulder arthroplasty (TSA) has been documented at 10% but may be as high as 20% (9). The most common complications of unconstrained TSA are glenoid loosening, instability, and rotator cuff tears. Each of these complications occurs in 1% to 2% of all unconstrained total shoulder arthroplasties. The majority of failed arthroplasties include two or more modes of failure—for example, glenoid loosening in the setting of instability (Fig. 14-1). Less common complications occurring in less than 1% of cases include intraoperative fractures, component malposition, nerve injury, infection, and humeral stem loosening. Sepsis is estimated to occur in less than 0.5% of unconstrained TSA cases.
Humeral head replacement (HHR) has a higher complication rate than TSA and approaches 16% (9). Complications after HHR vary depending on the preoperative diagnosis. HHR for osteoarthritis is more likely to be complicated by glenoid arthrosis, whereas HHR for proximal humerus fractures is more likely to result in tuberosity nonunion (10,11). In combined series, the most common complications of HHR occur in 1% to 3% of cases and include instability, glenoid arthritis, tuberosity nonunion, rotator cuff tears, nerve injury, and infection, in descending order. Poor postoperative rehabilitation and the use of uncemented humeral stems in HHR have been linked to failure of HHR (12).
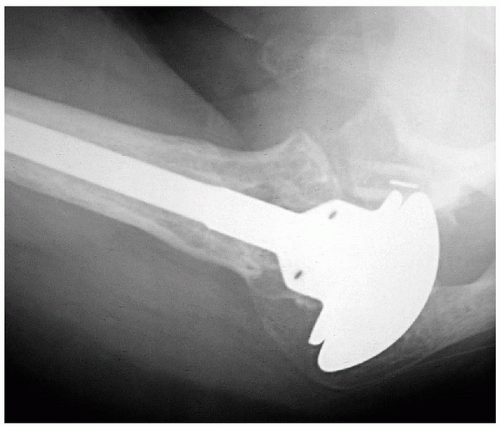 Figure 14-1 Axillary radiograph of a right shoulder, which reveals a posterior subluxated humeral head and a loose glenoid component. |
The complexity of the technical aspect of revision arthroplasty is the result of the combination of bone loss, muscle weakness, scar, and the greater threat of infection (1). The challenge in revision cases is working with and through compromised tissues. It has been noted that the success of shoulder arthroplasty is dependent on a functional myofascial sleeve more so than in total hip or knee arthroplasty (1,13). Therefore, unfortunately, even a wellexecuted revision arthroplasty can lead to a less-thansatisfactory result as a result of soft-tissue limitations. In any case, revision shoulder arthroplasty produces inferior, and often unpredictable results, when compared to primary shoulder arthroplasty (14,15).
The primary indication for revision shoulder arthroplasty is pain. Secondarily, loss of function or motion may be addressed with revision arthroplasty. Historically, the most common causes of failure of constrained or fixedfulcrum shoulder arthroplasty were glenoid loosening and dislocation of the prosthesis (4). Revision of the constrained arthroplasty was often complicated by insufficiency of the soft tissues and catastrophic bone loss. With the advent of unconstrained or “anatomic” TSA, the overall complication rate from TSA has decreased, but the modes of failure have increased (4,5). In particular, glenoid loosening with loss of bone stock and rotator cuff deficiencies continue to vex the shoulder surgeon. With the proliferation of shoulder arthroplasty, the need for revision surgery will continue to grow. It is imperative to scrutinize past failures and complications in an effort to improve techniques and, ultimately, outcomes in shoulder arthroplasty. The purpose of this chapter is to review the pathophysiology and the evaluation and treatment of failed shoulder arthroplasty.
SURGICAL ANATOMY
Normal
Understanding the normal anatomy and relationships in the glenohumeral joint is critical to performing an approximate anatomic reconstruction. With respect to articular reconstruction, there are proximal humeral and glenoid factors that must be considered (16,17). The size of the humeral head and its relationship to the humeral shaft are the most critical factors in reconstructing the proximal humerus. The average thickness of the humeral head is 19mm (2.4mm (Fig. 14-2). Either oversizing or undersizing the humeral head can lead to failure. Oversizing the humeral head or “overstuffing” leads to increased lateral offset and increased tension in the rotator cuff and deltoid. This may result in loss of motion or pain. Undersizing the humeral head may result in laxity of the rotator cuff and deltoid leading to instability. Similarly the superior aspect
of the articular surface has a consistent relationship to the greater tuberosity, usually 8 to 10 mm superior. Excessive height of the prosthesis may lead to overstuffing and loss of motion with possible rotator cuff failure. A proud greater tuberosity may lead to impingement. The version of the humeral prosthesis ideally should replicate the normal 30 to 35 degrees of retroversion of the proximal humeral articular surface. The version may be manipulated to obtain stability in revision surgery; however, failure to properly address the version may result in instability. The relationship of the humeral canal with respect to the cut or osteotomized portion of the proximal humerus is also crucial. The neck-shaft angle of the proximal humerus varies from 30 to 50 degrees. If the prosthetic system has a fixed head-neck angle, then the osteotomy should by made at that angle. More anatomic or modular systems may accommodate a variety of varus or valgus osteotomies. The anteroposterior humeral offset, defined by the distance between the axis of the medullary canal and the center of the humeral head, ranges from 0 to 4 mm (posterior), should also be reproduced. Most modern shoulder arthroplasty systems have offset head designs to address this issue. Ideally, the prosthetic humeral head should not overhang the cut surface. Malpositioning the prosthetic humeral head may lead to abnormal motion and/or bony contact.
of the articular surface has a consistent relationship to the greater tuberosity, usually 8 to 10 mm superior. Excessive height of the prosthesis may lead to overstuffing and loss of motion with possible rotator cuff failure. A proud greater tuberosity may lead to impingement. The version of the humeral prosthesis ideally should replicate the normal 30 to 35 degrees of retroversion of the proximal humeral articular surface. The version may be manipulated to obtain stability in revision surgery; however, failure to properly address the version may result in instability. The relationship of the humeral canal with respect to the cut or osteotomized portion of the proximal humerus is also crucial. The neck-shaft angle of the proximal humerus varies from 30 to 50 degrees. If the prosthetic system has a fixed head-neck angle, then the osteotomy should by made at that angle. More anatomic or modular systems may accommodate a variety of varus or valgus osteotomies. The anteroposterior humeral offset, defined by the distance between the axis of the medullary canal and the center of the humeral head, ranges from 0 to 4 mm (posterior), should also be reproduced. Most modern shoulder arthroplasty systems have offset head designs to address this issue. Ideally, the prosthetic humeral head should not overhang the cut surface. Malpositioning the prosthetic humeral head may lead to abnormal motion and/or bony contact.
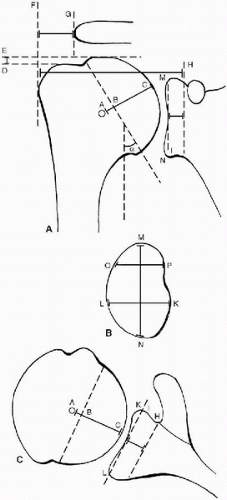 Figure 14-2 A, B, and C: Diagrams showing the anatomic measurements of the glenohumeral joint. The radius of curvature of the humeral head is represented by A-C in diagrams A and C. The thickness of the humeral head is represented by B-C in diagrams A and C. The neck-shaft angle is represented by α in diagram A. The lateral humeral offset is represented by F-H in diagram A. The distance from the greater tuberosity to the lateral acromial process is represented by F-G in diagram A. The distance from the humeral head to the greater tuberosity is represented by D-E in diagram A. The superior-inferior height of the glenoid is represented by M-N in diagrams A and B. The anteroposterior dimensions of the glenoid are represented by L-K in diagrams B and C and by O-P in diagram B. The joint line of the glenoid is represented by H-I in diagrams A and C. (Reprinted and modified from Iannotti JP, Gabriel JP, Schneck SL, et al. The normal glenohumeral relationships. J Bone Joint Surg 1992;74-A(4):492, with permission.) |
On the glenoid side, the two main factors over which the surgeon has control are the version and the offset. The normal version of the glenoid articular surface with respect to the scapula is 0 to 5 degrees of retroversion with slight superior inclination. Reconstruction in excessive retroversion or anteversion may result in posterior or anterior instability, respectively. The relationship of the glenoid articular surface to the base of the coracoid is fixed and is approximately 5 mm. Each arthroplasty system also has a standard thickness of glenoid prosthesis. Therefore, the lateral offset is determined by the size (and positioning) of the prosthetic humeral head and thickness of the prosthetic glenoid. Failure to understand these relationships may result in a painful, stiff joint.
Pathologic
Humerus
Humeral bone loss is often an issue in failed shoulder arthroplasty for proximal humeral fractures but may complicate any revision shoulder arthroplasty. Humeral bone loss may involve the tuberosities, the metaphysis, the diaphysis, or a combination of these. Loss of the tuberosities is a devastating complication in revision arthroplasty because the rotator cuff exerts its power through the tuberosities. In the setting of arthroplasty for proximal humeral fractures, the tuberosities may displace or, worse, resorb. It is imperative that the tuberosity unites to the humeral shaft to obtain a successful reconstruction. With no attachment site for the rotator cuff, a dysfunctional
or flail shoulder may be inevitable despite soft-tissue reconstruction.
or flail shoulder may be inevitable despite soft-tissue reconstruction.
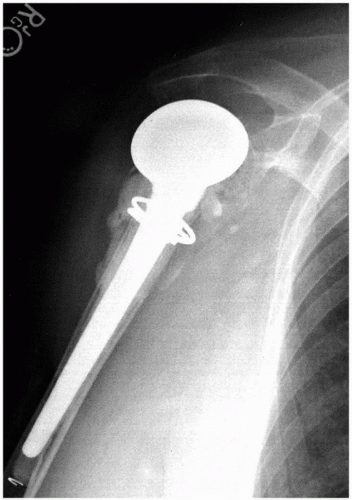
Metaphyseal and diaphyseal bone loss may complicate revision arthroplasty. The main issues with these deficiencies are humeral stem fixation and restoration of humeral height. In nonsegmental bone loss, the overall length of the humerus is not generally a problem. Fixation of the stem may be problematic in nonsegmental bone loss when the canal is patulous or the proximal trabecular bone is absent. In the majority of cases, the use of cement for humeral stem fixation provides a stable construct. Impaction bone grafting has also been described in the setting of shoulder arthroplasty, primary and revision, with some success (18,19). In this technique, cancellous bone graft is used to restore the cancellous bone stock for implantation of a press-fit or ingrowth stem. A long-stem prosthesis can be used to bypass any deficient area and obtain fixation more distally in better bone stock (Fig. 14-3) (20). This technique is useful in periprosthetic fractures and in revision cases where the cortex may have been perforated during cement removal. There may be cases—in young people, for example—where restoration of bone stock for an ingrowth prosthesis or possible later revision is desired. In this setting, a few options have been described. With cortical or peripheral loss of bone stock, extensive autograft or strut allograft fixed with cables or wires are good options. In either setting, it is imperative to achieve stable stem fixation at the time of the revision. Thus, in the absence of cement, a stable interference fit is required to prevent loosening of the humeral prosthesis. In older patients or patients with osteopenia, the risk of intraoperative periprosthetic fractures is theoretically increased with obtaining a stable press fit.
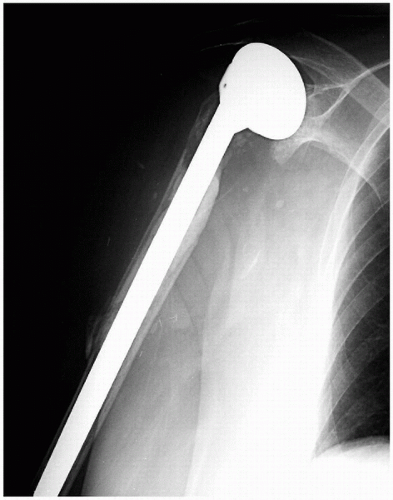 Figure 14-3 This anteroposterior radiograph of a right shoulder shows a long-stem humeral prosthesis used to bypass a small cortical defect, which occurred during cement removal. |
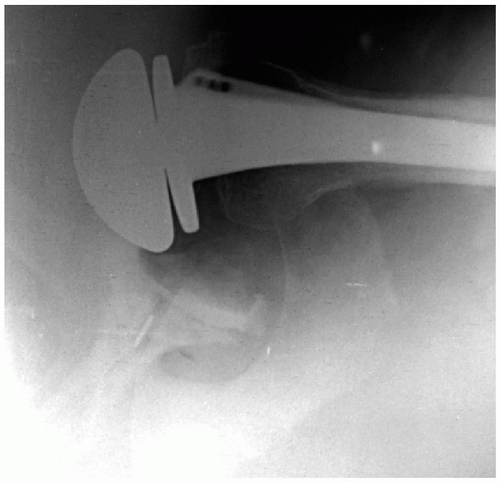
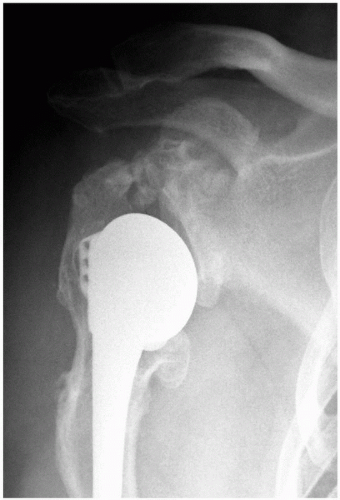 Figure 14-4 Anteroposterior radiograph of a right shoulder, which reveals relative humeral shortening as a result of a poorly positioned stem. |
In proximal humerus fractures or other settings of segmental bone loss, restoration of humeral length is crucial. Overlengthening can result in relatively “overstuffing” the joint and may lead to pain and loss of motion. Overlengthening the stem in arthroplasty for proximal humerus fractures can result in tuberosity failure (11). Shortening the proximal humerus can result in weakening of the deltoid lever arm, potentially leading to pain, loss of motion, and inferior instability (Fig. 14-4). In the setting of segmental humeral bone loss, there are a number of options available. A long-stem prosthesis can often reestablish the height while achieving stable fixation in the distal diaphysis. If a long-stem prosthesis is being used to
bypass a cortical defect, the tip of the chosen prosthesis should extend at least 2.5 cortical diameters distal to the defect (5). A structural allograft can be combined with the humeral prosthesis to restore the lost segmental bone stock as necessary. For more extensive bone loss, custom humeral prostheses or tumor-type bulk prostheses can be used. There is limited information about the use of these latter techniques in the setting of revision arthroplasty. Ideally, the least invasive or least complicated method effective in restoring the humeral loss should be preferred.
bypass a cortical defect, the tip of the chosen prosthesis should extend at least 2.5 cortical diameters distal to the defect (5). A structural allograft can be combined with the humeral prosthesis to restore the lost segmental bone stock as necessary. For more extensive bone loss, custom humeral prostheses or tumor-type bulk prostheses can be used. There is limited information about the use of these latter techniques in the setting of revision arthroplasty. Ideally, the least invasive or least complicated method effective in restoring the humeral loss should be preferred.
Glenoid
Glenoid bone loss is a significant problem with revision TSA, particularly in the setting of aseptic glenoid loosening. In the past, the use of constrained implants led to catastrophic glenoid deficiencies. As a result of this and other complications, the use of constrained implants has fallen out of favor. Nonetheless, glenoid bone deficiency remains an unsolved problem even with revision of more anatomic shoulder arthroplasty systems. Glenoid bone loss can occur through a number of mechanisms. Osteolysis is the most common mechanism of glenoid bone loss around a cemented glenoid. Macroscopically loose or displaced glenoid components can lead to mechanical wear and loss of bone stock. Excessive bone removal during the revision of a cemented or ingrowth component can also lead to glenoid deficiency. Glenoid deficiencies have historically been described as peripheral, central, or complex (peripheral and central) (6).
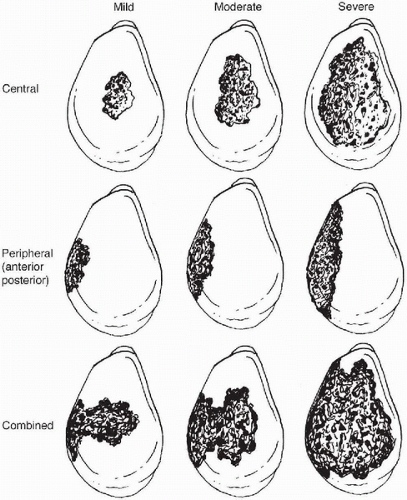 Figure 14-5 Classification of glenoid bone deficiencies after glenoid component removal. Mild and moderate deficiencies are often suitable for component reimplantation with or without bone grafting of the glenoid. Severe central or combined deficiencies often preclude implantation of a new component. (Reprinted from Antuna SA, Sperling JW, Cofield RH, et al. Glenoid revision surgery after total shoulder arthroplasty. J Shoulder Elbow Surg 2001;10(3):218, with permission.) |
In 2001 a quantitative classification scheme for glenoid bone deficiency in revision arthroplasty was proposed by Antuna and colleagues (Fig. 14-5) (2). On the basis of 43 revised glenoid components, glenoid bone loss was categorized
intraoperatively by location and severity. Location of the deficiency was either central, peripheral (anterior or posterior), or combined (peripheral and central). Severity was determined by the amount of glenoid rim involvement—less than 1/3 was mild, 1/3 to 2/3 was moderate, and greater than 2/3 was severe. Of the patients, 67% had isolated central deficiency, and the remainder of patients had combined deficiencies. They elected to reimplant a glenoid component in 25 shoulders of which 22 had mild or moderate deficiencies and only 3 had severe central deficiency with an intact glenoid rim. An uncemented, ingrowth component fixed with screws was used in 8 shoulders, 5 of which received cancellous allograft. A variety of glenoid components were cemented in the remaining 17 shoulders; only 1 of these received cancellous allograft. The remaining 18 shoulders had severe central and combined deficiencies that the surgeon felt precluded stable reimplantation. Of these, 15 shoulders received cancellous bone graft, and all but 1 was allograft. Ultimately, 12 of these patients obtained satisfactory pain relief. The remaining 6 were diagnosed with glenoid-sided pain, and 3 had been revised. Overall, satisfactory pain relief was obtained in 86% of the shoulders that underwent glenoid reimplantation versus 66% pain relief in the shoulders with glenoid removal. The authors suggest that replacement of a glenoid component is usually possible when central or peripheral bone deficiencies are limited or moderate. The authors noted that only 14.5% of these patients were revised for isolated glenoid loosening. Of these, 68% had additional modes of failure.
intraoperatively by location and severity. Location of the deficiency was either central, peripheral (anterior or posterior), or combined (peripheral and central). Severity was determined by the amount of glenoid rim involvement—less than 1/3 was mild, 1/3 to 2/3 was moderate, and greater than 2/3 was severe. Of the patients, 67% had isolated central deficiency, and the remainder of patients had combined deficiencies. They elected to reimplant a glenoid component in 25 shoulders of which 22 had mild or moderate deficiencies and only 3 had severe central deficiency with an intact glenoid rim. An uncemented, ingrowth component fixed with screws was used in 8 shoulders, 5 of which received cancellous allograft. A variety of glenoid components were cemented in the remaining 17 shoulders; only 1 of these received cancellous allograft. The remaining 18 shoulders had severe central and combined deficiencies that the surgeon felt precluded stable reimplantation. Of these, 15 shoulders received cancellous bone graft, and all but 1 was allograft. Ultimately, 12 of these patients obtained satisfactory pain relief. The remaining 6 were diagnosed with glenoid-sided pain, and 3 had been revised. Overall, satisfactory pain relief was obtained in 86% of the shoulders that underwent glenoid reimplantation versus 66% pain relief in the shoulders with glenoid removal. The authors suggest that replacement of a glenoid component is usually possible when central or peripheral bone deficiencies are limited or moderate. The authors noted that only 14.5% of these patients were revised for isolated glenoid loosening. Of these, 68% had additional modes of failure.
The presence of a peripheral glenoid deficiency presents a complex problem. In the setting of primary arthroplasty with glenoid deficiency, a number of techniques have been described with varying degrees of success (21, 22, 23). Basic scientific research has shown that preparation of the glenoid to match the contour of the glenoid implant results in increased stability of the implant and may theoretically decrease the risk of glenoid loosening (24). The optimal glenoid deficiency is an asymmetric wear pattern typically posterior with adequate bone stock to allow eccentric glenoid reaming. In this scenario, the anterior or high side of the glenoid can be reamed, resulting in a concentric glenoid. Conversely, an anterior wear pattern can be treated by eccentrically reaming the posterior glenoid. With the exception of failed hemiarthroplasty resulting from glenoid arthrosis, this scenario is rarely, if ever, encountered in revision shoulder arthroplasty.
A more significant problem is glenoid rim insufficiency that precludes stable fixation of the glenoid prosthesis. Neer and Morrison recommended that 80% of a metal-backed glenoid prosthesis and 100% of a polyethylene prosthesis be supported by good bone stock (21). Collins and colleagues found that in reamed glenoid bone 25% to 33% glenoid deficiency did not affect displacement of the prosthesis under posteriorly directed forces in their cadaveric study (24). Various materials have been described for the treatment of peripheral glenoid deficiencies including cement, allograft bone, autograft bone, and custom glenoid prostheses (Fig. 14-6) (21). The cement augmentation and custom glenoid prostheses were not found to be durable alternatives to the deficient glenoid. The use of bone grafts fixed with screws or heavy sutures is well described with reasonable results in some hands. The modest success of these latter techniques in primary arthroplasty has not translated into widespread use of these techniques in revision shoulder arthroplasty. Current clinical research seems to favor leaving the glenoid component out rather than reconstructing the glenoid rim at the time of revision shoulder arthroplasty (25). When the glenoid component is removed without revision, a glenoidplasty may be performed to provide a relatively concentric surface to support the hemiarthroplasty. Some authors recommend bone grafting central deficiencies in an attempt to restore bone stock for a potential future revision (2).
Soft Tissue
The soft tissues are the nonbony structures that contribute to the success or failure of a shoulder arthroplasty and include the shoulder capsule and supporting ligaments, rotator cuff muscles, coracoacromial ligament, and deltoid muscle. Abnormalities of the soft tissues including laxity, contractures, paralysis, adhesions, and loss of tissue may contribute individually or in combination to the failure of a shoulder replacement. Neer and Kirby have stated that paralysis or loss of both the rotator cuff muscles and deltoid is a contraindication to revision shoulder arthroplasty (1). There are a number of soft-tissue complications that do not preclude revision arthroplasty but make the task considerably more difficult.
Laxity of the soft tissues is infrequently cited as an isolated cause of failure for shoulder arthroplasty. In the setting of instability, often the prosthetic components are placed in improper version contributing to the capsular laxity (26, 27, 28, 29). Secondarily, the soft tissues opposite the laxity may become contracted over time. Correcting the laxity without addressing the malpositioned component and coexistent contracture is unlikely to result in satisfactory outcomes. Laxity of the rotator cuff and deltoid more commonly occurs in the setting of humeral bone loss from a proximal humerus fracture. Inadequately restoring the height of the humeral head or the overall length of the humerus can result in laxity of the musculotendinous cuff, leading to loss of power and, potentially, instability.
Soft-tissue adhesions are a significant problem in failed shoulder arthroplasty, particularly in a stiff and painful arthroplasty. Adhesions make the exposure in revision shoulder arthroplasty challenging. Once the deltopectoral interval is developed, the surgeon may find the strap muscles have formed adhesions with the underlying subscapularis. The rotator cuff is commonly adhered to the undersurface of the acromion. This plane should be carefully
exposed for both mobilization and inspection of the cuff. The more peripheral cuff and proximal humerus are often adhered to the overlying deltoid muscle.
exposed for both mobilization and inspection of the cuff. The more peripheral cuff and proximal humerus are often adhered to the overlying deltoid muscle.
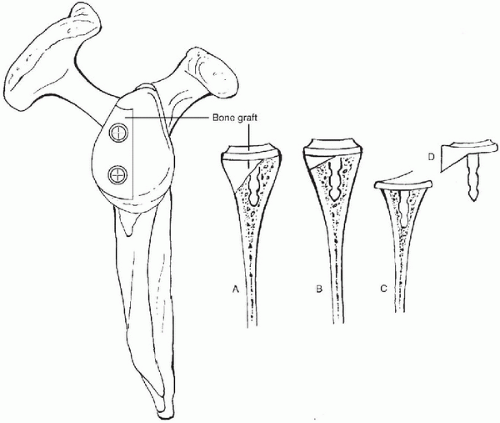 Figure 14-6 The methods of dealing with a deficient glenoid. A: Use of a large, internally fixed bone graft. B: Build-up of cement on the low side (not recommended). C: Reaming the high side to obtain proper version of the component (requires adequate bone stock). D: Use of an uneven, augmented glenoid component (not recommended). (Reprinted and modified from Neer CS II, Morrison DS. Glenoid bone-grafting in total shoulder arthroplasty. J Bone Joint Surg 1988;70-A(8):1157, with permission.) |
Contractures of the shoulder capsule and rotator cuff also complicate revision arthroplasty. Contractures typically occur in the setting of instability where one side, anterior or posterior, is lax or deficient and the opposing side is contracted. In anterior instability, the subscapularis is often deficient and the posterior capsule becomes contracted. In posterior instability, the subscapularis and anterior capsule may become contracted, whereas the posterior capsule becomes excessively lax. Global cuff and capsular contracture can occur in patients who received poor postoperative rehabilitation or with undersized humeral heads that develop postoperative adhesive capsulitis and stiffness. In this scenario, exposure may be exceedingly difficult. The operating surgeon must be diligent and methodic in releasing the capsule from the glenoid and mobilizing the rotator cuff.
Soft-tissue deficiency can be a devastating complication of shoulder arthroplasty. Deltoid deficiency, in particular, whether from axillary nerve damage or dehiscence, can obviate a satisfactory result in revision arthroplasty. Anterior-superior instability is another dire complication and is related to deficiency of the coracoacromial arch with or without deltoid insufficiency. In this population, the primary arthroplasty is performed in the setting of a known rotator cuff deficiency, typically combined supraspinatus and infraspinatus tears that are either irreparable or of poor tissue quality. Less commonly, the cuff deficiency occurs postoperatively. In the absence of a functioning rotator cuff, the fulcrum shifts superiorly to the superior glenoid and coracoacromial arch, whereas the deltoid provides the power for elevation. Although suboptimal, this scenario is not incompatible with a satisfactory result, as has been shown with the use of hemiarthroplasty for rotator cuff deficient shoulders. Success is predicated on a competent coracoacromial arch. Graft reconstruction of
the arch has been described in the setting of revision arthroplasty (27). More recently, redesigned reverse ball-and-socket prostheses have been used to address this problem with some success (30,31).
the arch has been described in the setting of revision arthroplasty (27). More recently, redesigned reverse ball-and-socket prostheses have been used to address this problem with some success (30,31).
In the primary and revision arthroplasty setting, a deficient superior or postero-superior rotator cuff are considered contraindications to glenoid implantation because of the high incidence of glenoid loosening as a result of altered shoulder mechanics (32). If the superior rotator cuff defect is reparable with good tissue quality, the surgeon may elect to repair the cuff and implant a glenoid. There is no current recommendation to perform a tendon transfer such as a latissimus transfer to restore superior or postero-superior rotator cuff lesions in the setting of revision shoulder arthroplasty.
Anterior capsular and subscapularis deficiencies occur in the setting of anterior instability. Anterior instability after shoulder arthroplasty is rarely a result of isolated soft-tissue deficiency but is more commonly associated with improper version of the glenoid or humeral head implants (26, 27, 28, 29). The subscapularis is more likely to be detached but may be attenuated. After revision of the components, the subscapularis can be primarily reattached to the proximal humerus if it is of good quality. Otherwise, the deficient soft tissues should be replaced using either an allograft or a tendon transfer (26,27). Achilles tendon allografting of the anterior glenohumeral joint to replace the deficient capsule and subscapularis has been described with some success (Fig. 14-7) (26). Additionally, the use of a pectoralis major tendon transfer has been described as a successful treatment for subscapularis insufficiency (27).
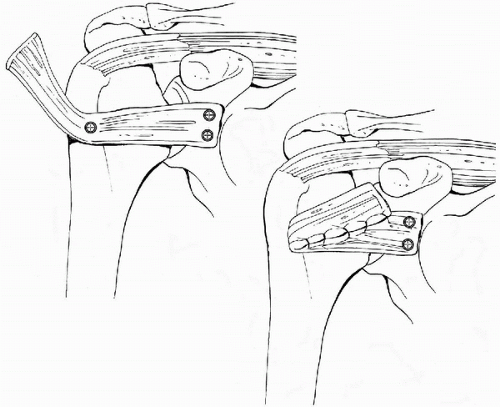 Figure 14-7 This drawing illustrates how the bone-Achilles tendon allograft can be secured to the glenoid neck and humeral to provide an anterior restraint in the setting of subscapularis insufficiency. (Reprinted and modified from Moeckel BH, Altchek DW, Warren RF, et al. Instability of the shoulder after arthroplasty. J Bone Joint Surg 1993;-75-A(4):495, with permission.) |
Prosthetic
Prosthetic malposition is another commonly identifiable cause of failure of shoulder arthroplasty (5,12,26,27). This mode of failure is an error of surgical technique because prosthetic positioning is under the operating surgeon’s control. On the humeral side, there are a number of anatomic factors that should be replicated to avoid arthroplasty failure. These factors include the height of the humeral prosthesis, the height of the humeral head, the offset of the stem (multiplanar), the offset of the humeral head (multiplanar), the size (both diameter and thickness) of the humeral head, and the version of the stem. Excessive height of the humeral prosthesis can result in loss of motion and pain. Insufficient height can result in deltoid weakness leading to loss of motion; pain; and, potentially, inferior instability. Errors in offset may alter the mechanics of the joint and lead to abnormal bone contact. Offset malpositioning can also contribute to overstuffing the joint. Undersizing the humeral head can lead to impingement of the greater tuberosity under the coracoacromial arch (Fig. 14-8). Oversizing the humeral head can lead to stiffness and may result in soft-tissue deficiencies such as rotator cuff tears (Fig. 14-9). Errors in version are commonly identified as the cause of instability after shoulder arthroplasty (26, 27, 28, 29). Anterior or posterior instability can
occur depending on the amount of excessive anteversion or retroversion of the humeral prosthesis, respectively (Fig. 14-10). Version errors can occur in isolation, either proximal humeral or glenoid sided, but may often occur in combination.
occur depending on the amount of excessive anteversion or retroversion of the humeral prosthesis, respectively (Fig. 14-10). Version errors can occur in isolation, either proximal humeral or glenoid sided, but may often occur in combination.
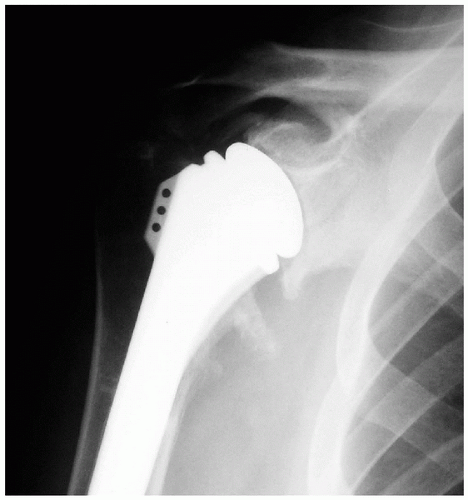 Figure 14-8 Anteroposterior radiograph of the right shoulder in a patient with pain and stiffness after revision shoulder arthroplasty that reveals an undersized humeral head. |
Malpositioned stems that are well fixed can be a source of frustration to the surgeon. If the system is modular and the problem can be corrected without removing the stem, there is no reason to revise the stem. If the position of the stem is unacceptable and is unable to be extracted by standard means, a controlled corticotomy is performed in the interval between the pectoralis major and deltoid insertions on the anterior humerus. The use of a long-stem prosthesis and cerclage wiring is recommended for stable reimplantation.
Glenoid malposition is usually an error of version. Excessive retroversion or anteversion of the glenoid component can result in posterior or anterior instability, respectively (Fig. 14-11). Additionally, the glenoid prosthesis contributes to the overall lateral offset, but, because the thickness of any given glenoid prosthesis is constant, overstuffing is a result of proximal humerus malpositioning or humeral head sizing. Failure to center the glenoid prosthesis in the vault can result in perforation of the glenoid wall, leading to cement extravasation and, potentially, glenoid loosening.
PATHOPHYSIOLOGY
Definition and Classification
The assessment of a shoulder arthroplasty as a failure can be a complicated issue. Patient’s expectations for and satisfaction with a shoulder arthroplasty may be the ultimate test of the success or failure of a shoulder arthroplasty. Hasan and colleagues in 2002 published a study suggesting that the revision rate in shoulder arthroplasty underestimates the failure of the procedure (33). The characteristics of 139 patients who were evaluated for dissatisfaction with their shoulder arthroplasty were examined. In this study, 74% of the shoulders were stiff; 35% were unstable; and, of the total shoulder replacements, 59% of the glenoid components were loose. Ultimately, 23% of the failures were not revised. Despite these findings, standard outcome assessments that include both function and pain components are a clinician’s best measure of the success of the procedure. Patient expectations are best addressed preoperatively through informed consent. In any case, a shoulder arthroplasty that fails to give a patient adequate pain relief is a failure by any measure.
We propose a simple classification scheme for failed shoulder arthroplasty based on the nature of the failure. Extrinsic failure includes all the diagnoses that can be treated without revision of prosthetic components. Examples of extrinsic failure include impingement syndrome, nerve damage, deltoid failure, symptomatic acromioclavicular joint arthritis, rotator cuff tears, postoperative adhesive capsulitis, and instability not related to poor implant position. Intrinsic failures include those failures related to prosthetic components and whose treatment requires implant revision. Glenoid arthrosis would be considered an intrinsic failure because a glenoid component is required for treatment. Examples of intrinsic failure include instability with glenoid loosening, instability with poorly positioned components, aseptic glenoid loosening, aseptic humeral loosening and any failure related to poor implant choices or fixation. To better study and report revisions, three factors at the time of revision surgery are considered: glenoid revision, proximal humerus revision, and soft-tissue deficiency. There is evidence that revision arthroplasty involving only glenoid-sided revision, for example, has a better overall outcome than other revisions. This classification would remove the complexity of defining the causes for failures, which are often multifactorial and make classification burdensome. By comparing similar procedures, clinicians can provide more useful and comparable data. In this classification scheme, joint sepsis is considered extrinsic and is classified as either early (within 4 weeks of surgery) or late.
Pathogenesis
The pathogenesis of aseptic failure in shoulder arthroplasty is a topic of great concern to the shoulder surgeon. Since the inception of TSA, the fate of the glenoid component has received more attention than any other aspect of shoulder arthroplasty. In a comprehensive review of published series of TSAs, Rodosky and Bigliani reported the failure rate of the glenoid component as defined by revision of the glenoid component as ranging from 0% to 12.5%, depending on the study and the type of prosthesis used (Fig. 14-12) (34). The authors recognized the relatively short-term follow-up of 3 to 5 years for the majority of the studies. Based on long-term follow-up data, Torchia and colleagues estimated component survivorship of TSA to be 93% and 87% at 10 and 15 years, respectively (35). Overall, these numbers seem to represent satisfactory retention rates for the glenoid component. The more alarming issue is the presence and potential significance of radiolucent lines around the glenoid component. In the same study, Rodosky and Bigliani reported an incidence of radiolucent lines to vary from 26% to 100%. Perhaps more concerning was the progression of the radiolucent lines, which varied from 0% to 36%. Glenoid radiolucent lines have been documented at high rates on early postoperative radiographs and have been linked to fixation technique (36). Further study has shown that improved cementing technique lowers the incidence of postoperative radiolucent lines (21). The relationship of radiolucent lines to glenoid component failure remains unclear and is a matter of ongoing debate.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree