Revision Elbow Arthroplasty
Gordon Beadel
Graham King
INTRODUCTION
Over the last few years the indications for total elbow joint replacement have expanded from inflammatory arthritis, especially rheumatoid arthritis, to include osteoarthritis, posttraumatic arthritis, fractures, nonunions, and tumors. Some of these indications have been associated with a higher rate of implant failure. As the number of primary total elbow joint replacements has increased, there has been an associated increase in the number requiring revision for failure.
In this chapter the various mechanisms of failure, evaluation of the failed implant, and techniques for revision surgery are discussed. Revision total elbow joint replacement is a technically challenging procedure associated with high complication rates. Ideally, the surgeon should have considerable experience with primary elbow arthroplasty before attempting a revision. The functional results of a successful revision arthroplasty are superior to the alternatives, which include excisional arthroplasty and arthrodesis; therefore revision should almost always be considered in the setting of a failed arthroplasty.
MECHANISMS OF FAILURE
An elbow joint replacement may fail acutely or insidiously. Sudden dramatic failure may be the result of infection, periprosthetic fracture, component failure including implant fracture or uncoupling, or dislocation. Slower modes of failure include aseptic loosening, polyethylene bearing wear, instability of an unlinked implant, and chronic lowgrade infection.
Infection
The diagnosis of infection as the cause of implant failure must always be considered, and as best able confirmed or excluded, prior to proceeding with revision arthroplasty. Three main factors have been found to influence the outcome of an infected elbow replacement: the organism involved, the fixation of the implant, and the time interval between onset of symptoms and treatment. An infected elbow arthroplasty is a devastating complication with no easy cure and requires a different treatment plan than that for other mechanisms of failure.
The most common infecting organisms are Staphylococcus aureus and Staphylococcus epidermidis (1, 2, 3, 4, 5, 6), but a wide range of others have also been implicated including Enterococcus, Pseudomonas, β-hemolytic Streptococcus, and Klebsiella. Infections resulting from S. epidermidis have been associated with a high incidence of recurrent infection requiring excisional arthroplasty for management (3). This may be the result of the ability of S. epidermidis to produce protective biofilms.
The state of fixation of the implant to the bone is also important. Aggressive debridement and irrigation alone are more likely to be successful in eradicating infection if the implant is well fixed than if the implant is loose (3). Because of the high risk of substantial bone loss and fracture associated with removal of a well-fixed infected implant, an initial attempt to retain the components with irrigation and debridement is usually appropriate. If the prosthesis is loose, then debridement alone has poor results and the recommended management is removal of the implant and debridement with subsequent staged reimplantation if possible.
The third important factor is the timeframe between onset of symptoms and treatment. Traditionally, infection has been categorized by the time interval between the index procedure and diagnosis as acute (<3 months), subacute (between 3 months and 1 year), or late (>1 year). However, this has not been shown to be associated with outcome (3). Infection is better defined by the interval between onset of symptoms and treatment, as either acute or chronic. It is generally agreed that symptoms of less than 30 days represent an acute infection, and symptoms of longer duration represent a chronic infection. With infection other than S. epidermidis, prosthesis retention with extensive debridement and irrigation in the presence of a well-fixed implant is more likely to be successful with acute as opposed to chronic infection (3,6).
Acute joint sepsis can present in the immediate postoperative period or many years later when seeded hematologically from a focus elsewhere in the body. Systemic symptoms may be present along with joint pain, erythema, swelling and induration, decreased range of motion, and wound drainage. Surgical wound drainage for more than 48 hours postoperatively is highly suspicious of a deep infection and should be treated aggressively with debridement, irrigation, and antibiotics. More commonly, infection presents insidiously with gradually increasing pain, present at rest and at night; swelling; loss of motion; and progressive radiologic changes of implant loosening.
Reported infection rates following primary elbow arthroplasty vary from 1% to 12.5% (3,5,6,8,9,16,19, 20, 21). The infection rates are generally higher than those for total hip or knee replacement; this is thought to be the result of the subcutaneous nature of the elbow joint with little soft-tissue coverage. Significant risk factors for infection include a history of previous elbow operation or infection, psychiatric illness, class IV rheumatoid arthritis, postoperative wound drainage, spontaneous wound drainage after postoperative day 10, and elbow reoperation for any reason (4,6). Other factors that also have been associated with infection include psoriatic arthritis and immunocompromise secondary to illness, medication, or diabetes. Patients with more than one factor have an even greater risk of infection (e.g., the incidence of infection in a patient with rheumatoid arthritis who has had previous elbow surgery has been reported to be as high as 31%) (6).
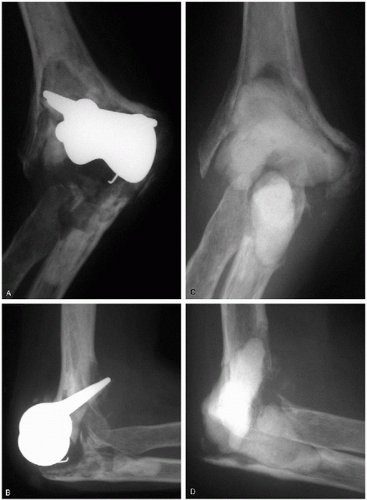
Options for treatment of an infected arthroplasty include long-term antibiotic suppression, debridement and prosthesis retention, excisional arthroplasty, excisional arthroplasty, and immediate or delayed reimplantation or arthrodesis as a two-stage procedure (3,4,6,9,20). With a well-fixed prosthesis and acute infection (symptom duration less than 30 days) an attempt at eradication of infection and implant retention by undertaking multiple joint debridements is usually appropriate (3). Joint debridement includes implant disarticulation, debridement of necrotic tissue and debris, polyethylene bearing exchange, thorough irrigation, and insertion of antibiotic impregnated cement beads (the antibiotic being determined by the sensitivities of the organism). Intraoperative cultures should be taken at the time of each debridement, which should be repeated every 2 to 4 days. Typically at least three debridements are required and occasionally more (3), as guided by the clinical appearance of the elbow and the results of serial cultures and blood tests including white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP). If this is unsuccessful in controlling the infection, then excisional arthroplasty is indicated with the possibility of later reimplantation. With a loose prosthesis but adequate bone stock, implant removal and insertion of a temporary antibiotic impregnated cement spacer should be considered. Staged reimplantation can then be attempted when the infection has been eradicated (3). Single-stage debridement and prosthetic exchange is not recommended because of the higher rate of recurrent infection (3). If the infecting organism is S. epidermidis, then eradication of infection is most likely to be achieved by excisional arthroplasty without reimplantation (3,6). Excisional arthroplasty is recommended with persistent or recurrent infection and in patients in whom bone stock is too poor to contemplate reimplantation or who medically are too unwell to tolerate multiple surgical procedures (Fig. 29-1). The functional results following excisional arthroplasty are variable, with many patients requiring bracing for stability (2,3). Parenteral antibiotics based
on the sensitivities of the cultured organisms are usually administered for a minimum of 6 weeks for each of these options. Long-term oral antibiotics for chronic suppression may be required if there are concerns of residual infection. Multiple debridements through a poor soft-tissue envelope about the elbow may result in wound problems requiring the assistance of a plastic surgeon (7).
on the sensitivities of the cultured organisms are usually administered for a minimum of 6 weeks for each of these options. Long-term oral antibiotics for chronic suppression may be required if there are concerns of residual infection. Multiple debridements through a poor soft-tissue envelope about the elbow may result in wound problems requiring the assistance of a plastic surgeon (7).
Aseptic Loosening
Aseptic loosening is the most frequent cause of long-term implant failure (7, 8, 9). Presentation is usually insidious in a prosthesis that previously was well functioning, with gradual onset of activity-related pain, which becomes a more constant feature and limits function. The incidence is higher with the more constrained prostheses and in patients who continue to lift and undertake strenuous activity with the limb. Polyethylene wear debris results in macrophage-induced osteolysis at the bone cement or bone implant interface and progressive bone loss. With severe osteolysis, periprosthetic fractures can occur with minimal trauma (10), presenting acutely with a sudden increase in pain and deformity. Patients with asymptomatic periprosthetic osteolysis and implant loosening need to be followed regularly with radiographs to monitor the rate of progression and to determine when and if revision is indicated.
The incidence of aseptic loosening after total elbow replacement is related to a number of variables including the indication for surgery, the type of implant used, and the level of patient activity (10). The incidence of aseptic loosening is considerably higher, and the time to revision is significantly shorter in the setting of posttraumatic arthritis compared to rheumatoid arthritis (8, 9, 10,20,21). An early report from the Mayo Clinic found a 19% revision rate for aseptic loosening following posttraumatic arthritis at a mean follow-up of 6.3 years (8). This compares with a revision rate for aseptic loosening of 2.6% at 10 years in rheumatoid arthritis at the same institution (21). More recently they reported a 0% revision rate for aseptic loosening in posttraumatic arthritis, at a mean follow-up of 5.8 years (16). However, in this group, the revision rate for mechanical failure was high, including 12% for fracture of the ulnar component and 5% for polyethylene bearing wear. Kraay and colleagues (9) reported cumulative survival rates of total elbow replacements for posttraumatic arthritis, fracture, or nonunion of 73% at 3 years and 53% at 5 years, compared with 92% at 3 years and 90% at 5 years for inflammatory arthritis, for which implant survival was 97% at 5 years with respect to aseptic loosening alone. The rate of aseptic loosening in posttraumatic arthritis is higher in patients younger than the age of 60 years (8). Trail and colleagues (22) reported a revision rate for aseptic loosening of 13% after 12 years in a rheumatoid population, with 75% of these being the result of tilting and loosening of the humeral component. Constrained implants are associated with high early rates of loosening (8,10,23,24).
Periprosthetic Fracture
Fractures about a total elbow joint replacement may occur at the time of implant insertion, secondary to aseptic loosening and osteolysis (10) or as a consequence of a single traumatic event such as a fall. In the management of periprosthetic fractures, the fracture location, implant fixation, and quality of the remaining bone stock must be considered.
Intraoperative fractures are typically minimally displaced and if suspected should be confirmed at the time with radiographs. Fractures about the humeral condyles or epicondyles associated with implant insertion are often undisplaced and heal satisfactorily without internal fixation. Displaced epicondylar and small condylar fractures should be excised and the soft tissues should be repaired. Larger fragments should be reduced and internally fixed, especially in the setting of an unlinked arthroplasty where ligament attachment is required for joint stability. Shaft fractures are often spiral as a result of torque applied to the arm and can be managed with cerclage wiring and a longer stemmed implant, bypassing the fracture by at least two cortical diameters. Olecranon fractures should be managed with tension band wiring.
Periprosthetic fractures associated with loosening or a traumatic event are usually displaced and associated with severe pain, deformity, and instability with crepitus (11). Humeral shaft fractures proximal to a well-fixed implant can be treated nonoperatively with functional bracing; however, any residual deformity may affect implant longevity and make future revision elbow arthroplasty difficult. Therefore, the authors advise open reduction and internal fixation for many of these fractures. Olecranon fractures are not uncommon, particularly among patients with rheumatoid arthritis who have poor bone stock; they often can be treated nonoperatively if the periosteal sleeve and triceps mechanism remain intact. Nearly all the remaining fractures require operative intervention. If the component remains well fixed, then retention of the implant and fracture fixation with or without autologous bone grafting and/or strut allografts should be considered. If the implant is loose or fixation is significantly compromised, then the implant should be revised at the time of fracture reduction and fixation. The implant stem should bypass the fracture by at least two cortical diameters, and strut allograft and cancellous autograft should be used as required (11). The rates of postoperative periprosthetic fracture vary from 6% to 22% (5,19,20).
Implant Material Failure
Implant failure is usually a chronic process but occasionally presents acutely with fracture of the implant stem or failure of the linkage mechanism. More commonly there is progressive wear of the polyethylene bearing surface, resulting in excessive joint laxity, which in turn leads to further wear and potential polyethylene fracture and metal-on-metal articulation. This may present as progressive joint instability with discomfort, “squeaking,” or dislocation of the prosthesis if
the arthroplasty design relies on polyethylene to stabilize the elbow (12, 13, 14, 15). Factors thought to play a role in increased bearing wear include significant preoperative deformity, strenuous activity and lifting by the patient, and malalignment of linked prostheses, all of which result in excessive joint torque (13,16,17). Wear debris contributes to progressive osteolysis and aseptic loosening of the implant.
the arthroplasty design relies on polyethylene to stabilize the elbow (12, 13, 14, 15). Factors thought to play a role in increased bearing wear include significant preoperative deformity, strenuous activity and lifting by the patient, and malalignment of linked prostheses, all of which result in excessive joint torque (13,16,17). Wear debris contributes to progressive osteolysis and aseptic loosening of the implant.
Although rare, implant stem fracture is a recognized complication that is related to implant design and malalignment, excessive patient activity, and traumatic events (10,16, 17, 18, 19, 20, 21). Osteolysis may predispose to stem fracture as a result of compromised stem support, giving rise to a greater bending moment and thereby fatigue failure. The bearing mechanism may also fail acutely with backing out or breakage of the axis pin resulting in dislocation (2,5,7,9,18). In these circumstances presentation is usually dramatic with sudden onset of deformity, joint instability, loss of motion, and pain. It may be associated with a single traumatic episode.
Instability
Failure as a result of instability is usually seen with unlinked resurfacing elbow designs, where it is one of the more common complications, with an incidence ranging from 0% to 13% (17). Instability may also be seen in linked designs with progressive wear of the polyethylene that is used to maintain the linkage mechanism. Instability may be classified as immediate (perioperative), early (<6 weeks postoperative), or late (>6 weeks postoperative) (17).
Immediate or acute instability typically presents with dislocation that is easily diagnosed clinically and radiologically. It is usually the result of component malposition or triceps tendon or collateral ligament disruption, either at the time of surgery or by progressive attrition with activities of daily living. Late instability may present with dislocation but more commonly with insidious symptoms such as weakness, pain, giving way, clunking, or other mechanical symptoms. This may be secondary to component wear or malalignment or following a traumatic episode with ligament injury (17).
EVALUATION
Clinical Evaluation
Clinical evaluation begins with the history, including the original indication for total elbow joint replacement and the time to presentation from the index procedure. It is important to note that the surgeon needs to know if there were any early postoperative problems such as unexplained fever, prolonged wound drainage or breakdown, reoperation for wound problems, or courses of antibiotics. A history of any of these or an elbow joint that has never settled with regard to pain and swelling following the index procedure is suggestive of chronic deep infection. Current symptoms, especially those of pain, including aggravating and relieving factors, and mechanical instability should be assessed. Elbow function and limitations of activities of daily living, work, and hobbies are also evaluated. Neurovascular symptoms, particularly relating to the ulnar nerve, must be sought after. An overall assessment of the patient’s health, medical problems that may have contributed to the failure, regular medications, and fitness for further surgery should be performed.
On physical examination the soft-tissue envelope about the elbow joint including previous incisions and its suitability for further surgery need to be assessed. Included in this are markers to suggest infection: erythema, increased warmth, swelling and induration, wound drainage, scars to suggest previous sinuses, and a large joint effusion. A careful inspection of the alignment of the elbow and the range of motion including flexion, extension, pronation, and supination should be performed. Valgus, varus, and posterolateral rotatory instability tests should be performed looking for excessive laxity, abnormal joint tracking, subluxation, or frank dislocation (17). An axial push-pull test is used to evaluate bearing wear and movement of the components within the medullary canals. Motor strength about the elbow must be assessed, in particular the competency of the extensor mechanism. The ulnar nerve needs to be palpated, its course identified as far as possible, and its irritability assessed by way of a Tinel’s test. The distal neurovascular function of the limb needs to be carefully documented. Shoulder range of motion should be assessed to ensure that the patient can be positioned appropriately during surgery. Shoulder scars may also raise the suspicion of an underlying proximal humeral prosthesis, which may dictate the humeral implant stem length at the time of revision elbow surgery. Finally, for revision arthroplasty to be worthwhile, hand and wrist function must be adequate and therefore should be evaluated.
The patient’s clinical notes and operative report from the original surgeon should be obtained if at all possible. These may contain important information with regard to the indication for surgery, the surgical approach and management of the ulnar nerve, the implant type and size, and any early postoperative complications.
Imaging Studies
Plain anteroposterior and lateral radiographs of the elbow, humerus, and forearm are usually all that are required. In selected cases oblique radiographs may provide further information with regard to possible canal perforation or cement extrusion. Computed tomography (CT) scans typically have too much metallic artifact to be of much use, but this may change with improved technology and they may become more useful in defining the extent of implant loosening and bone loss.
It is helpful to have previous x-rays available for comparison so that more subtle progressive loosening or change in component position can be identified. The radiographs are scrutinized for signs of implant loosening and osteolysis as well as cortical perforation, cement extrusion, implant alignment, bony deformity resulting from previous fracture, previous internal fixation, or shoulder prostheses—all of which may affect the revision procedure and ultimately the component stem length.
Radiographic signs of aseptic loosening include radiolucent lines between the bone-cement interface, the cementimplant interface, or the bone-implant interface in the case of an uncemented prosthesis. Cracks in the cement mantle or a shift in the position of the implant are definitive evidence of loosening. Gross loosening is usually associated with severe osteolysis. Radiographic loosening may be graded by the bone-cement interface on the lateral radiograph (8). Type I is a radiolucent line less than 1-mm wide and involving less than 50% of the interface; Type II is a line more than 1-mm wide and involving more than 50% of the interface; Type III is a line more than 2-mm wide around the entire implant; and Type IV is gross loosening. Although a grading system like this is useful for documenting the radiologic appearance, it is more important to be able to recognize progressive changes because it is these cases that typically require operative intervention. Stable radiographic changes can often be followed closely and observed.
The extent and pattern of bone loss needs to be evaluated so that appropriate decisions can be made regarding the need for allograft bone and the type and length of revision implant required. Bone loss can be graded as suggested by Morrey and colleagues (8). Grade I is when subchondral architecture remains intact; Grade II is when the medial and lateral supracondylar columns are preserved without the trochlea; Grade III is when either the medial or lateral supracondylar columns are absent; and Grade IV is when the entire distal humerus to or proximal to the olecranon fossa is absent. The ulna can be graded as to whether or not the olecranon is present.
The location and extent of a periprosthetic fracture in relation to the implant needs to be clearly identified to allow treatment planning in these difficult cases. They may be classified according to the system of O’Driscoll and Morrey (26). A Type I fracture involves the humeral columns or condyles, the olecranon or the coronoid process. A Type II fracture is around the stem or at the tip of the implant, and a Type III fracture involves the shaft of the bone beyond the prosthesis. Periprosthetic fractures should be further subdivided as to whether or not the implant is loose because this is an important factor in their management.
In cases of suspected joint instability, when clinical examination and plain radiographs are nondiagnostic, fluoroscopy may be helpful in establishing the diagnosis (17). Arthrograms can be used to help evaluate stem loosening in equivocal cases.
Technetium- and indium-labeled white cell scans have been used in the diagnosis of infection but have not been particularly helpful because of the high false-positive rate with aseptic loosening.
Other Studies
Unfortunately no test is 100% sensitive or specific for periprosthetic infection. Prior to any revision, blood tests including a WBC count, ESR, and CRP, as markers for infection, should be routinely performed. The WBC count is unlikely to be elevated in chronic infection but may be elevated in an acute septic presentation (6). In the early postoperative period it is normal for both the ESR and CRP to be elevated. The CRP is the first to return to normal, usually by 3 weeks following the procedure, and the ESR significantly later, often months. If these tests are elevated outside this postoperative period, then this is suggestive of infection or inflammatory arthritis. Aspiration arthrography of the elbow joint is the most definitive test and should be performed prior to surgery if there is clinical suspicion of infection or if the CRP or ESR are elevated. Ideally this should not be performed within 4 weeks of a course of antibiotics because this increases the risk of a false-negative result. The joint fluid is sent for a cell count, gram stain, and culture. It is important not to forget the rarer causes of joint infection such as fungi, particularly in patients who are immunocompromised. There are no specific figures available for the elbow joint, but if the synovial white cell count is greater than 25,000/mm3 or the differential shows greater than 75% polymorphonuclear leukocytes (PMNs), then this suggests periprosthetic infection (27). Although a positive aspiration is almost certainly confirmatory of infection, a negative aspiration unfortunately does not exclude infection because of the high false-negative rate.
Intraoperatively, further specimens should be taken for gram stain, culture, and frozen sections. There is also a high false-negative rate for gram stains, however, a positive test is again strong evidence of infection. Intraoperative frozen sections can also be used to diagnose infection, as has been demonstrated in lower limb arthroplasty, with more than ten PMNs per high-power field being very suggestive of infection (28).
OPERATIVE MANAGEMENT
Indications
Operative intervention is usually indicated for symptomatic aseptic loosening, instability, implant failure, and infection. It should also be considered for progressive or extensive osteolysis even in the absence of symptoms. This is to avoid complications such as periprosthetic fracture; it is also because extensive bone loss makes revision surgery more difficult and increases the risks of cortical perforation, fracture, and cement extrusion.
Nonoperative management may be appropriate in selected cases because of medical illness or elbow pathology (e.g., a poor soft-tissue envelope, which precludes further surgery). In these circumstances a hinged brace may be beneficial. If the soft-tissue envelope is unsatisfactory, then a plastic surgeon should be consulted about the possibility of improving the soft tissues prior to proceeding with implant revision. This will most likely take the form of a local rotation or pedicle island flap (e.g., radial forearm flap) or a free microvascular flap.
Surgical options depend on the indications but include ligament reconstruction, bearing exchange, revision arthroplasty, excisional arthroplasty, and arthrodesis. Interposition arthroplasty is rarely indicated. Allograft elbow reconstruction in the situation of massive bone loss has been reported; however, the complication rates are extremely high and include fracture, nonunion, infection, instability, stiffness, graft resorption, and degenerative joint changes (29,30). Foulkes and Mitsunaga reported on two cases with an average postoperative arc of motion of only 55 degrees (29). We feel that massive bone loss can be better managed by revision elbow replacement with or without allograft supplementation or impaction grafting.
Patient Positioning
Revision of the ulnar component and bearing exchange are easily accomplished with the patient in the supine position, a sandbag beneath the shoulder and the arm on a bolster across the chest. The lateral decubitus position is used for complex humeral revisions, and the arm is placed over a well-padded bolster. This position allows for an extensile exposure of both the humerus and the ulna if required for management of cement removal, fractures, and cortical defects. The iliac crest is always prepared. A sterile tourniquet is used and positioned as high as possible on the arm. This is deflated after 2 hours, but it is reinflated after an appropriate interval if bleeding is problematic.
Surgical Exposure
Ideally the revision is performed through a posterior midline longitudinal skin incision, which protects the medial cutaneous nerves; however, previous skin incisions must be respected and used as required. Typically they need to be lengthened for adequate exposure. Minimal full-thickness medial and lateral skin flaps are elevated off the underlying deep fascia.
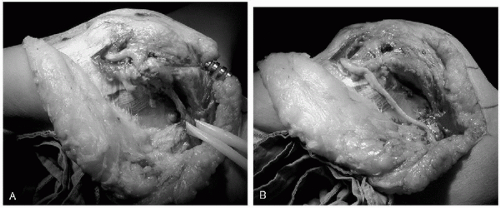
The ulnar nerve must always be identified and protected during the procedure (Fig. 29-2). If previously transposed and remaining anterior to the medial epicondyle, then it does not need to be widely dissected, but its course must be clearly identified to allow adequate protection. Otherwise an anterior subcutaneous transposition is always performed even in the absence of ulnar nerve symptoms (15). This is to protect the nerve from irritation by the implant and to decrease the risk of postoperative ulnar neuritis. It also increases the safety of the nerve if further revision surgery is required. The anterior subcutaneous pocket for the nerve to be positioned in at the end of the procedure is prepared in advance.
We typically expose the joint through the posterior midline triceps split described by Campbell (33). As previously discussed, the advantage of this approach is that it is extensile proximally, allowing identification and protection of the radial nerve in the spiral groove during implant and cement removal from the humerus and reimplantation (Fig. 29-3). This decreases the risk of damage by cutting tools or heat from extruded cement through an unsuspected cortical perforation (15).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree