Normal Anatomy
Although glenohumeral anatomy is described in detail in
Chapter 2 of this text, certain points relevant to replacement for osteoarthritis and AVN will be emphasized here.
The central 80% of the humeral head is spherical, and the peripheral 20% is elliptical (
7). However, if one assumes that the entire articular surface is spherical, humeral head radius of curvature, humeral head thickness, retrotorsion with respect to the humeral shaft, and neck-shaft angle are extremely variable (
7,
8,
9,
10). Mean humeral head radius is approximately 24 mm, with a range of 19 to 28 mm (
7,
10,
11). Mean humeral head thickness is approximately 19 mm, with a range of 15 to 24 mm (
7,
10,
11). Both humeral head radius and thickness correlate strongly with humeral shaft length and patient height (
7). However, the ratio of humeral head thickness to humeral head radius of curvature is remarkably constant at approximately 0.7 to 0.9, regardless of patient height or humeral shaft size (
7,
10,
11). The surface arc of the humerus available for contact with the glenoid is directly proportional to the ratio of humeral head thickness to humeral head radius and is, therefore, also relatively constant (
11).
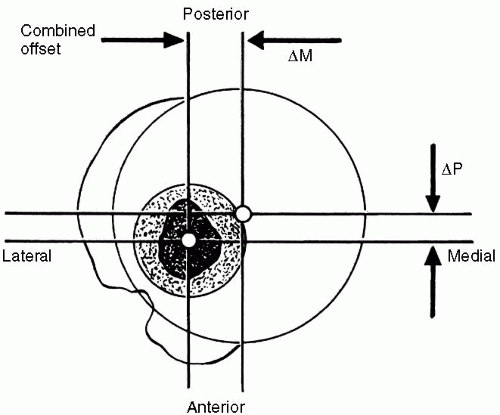
The distance between the center of the humeral head and the central axis of the intramedullary canal is defined as the humeral head offset (
8,
11,
12). Although humeral head offset is undoubtedly three-dimensional, it is commonly described in two planes, coronal and axial. Similar to most other proximal humeral anatomic parameters, reported humeral head offsets are variable (
8,
11,
12). In the coronal plane, the humeral head offset is approximately 7 to 9 mm medial to the central axis of the intramedullary canal; in the axial plane, the humeral head offset is 2 to 4 mm posterior to the central axis of the intramedullary canal (
Fig. 7-1) (
8,
11,
12).
Humeral head offset is correlated with humeral head radius and humeral head thickness. However, for a given humeral head radius, humeral head thickness, and humeral head offset in the coronal and axial planes, the location of the humeral articular surface may vary relative to angle of rotation about the central intramedullary axis (i.e., humeral retrotorsion). Humeral retrotorsion averages 20 to 30 degrees, with a wide range of approximately 20 to
55 degrees (
8,
10,
11,
12). The vertical distance between the highest point of the humeral articular surface and the highest point of the greater tuberosity (i.e., head to greater tuberosity height) is approximately 8 mm and shows a relatively small range of interspecimen variability (
7,
11).
The neck-shaft angle is defined as the angle subtended by the central intramedullary axis of the humeral shaft and the base of the articular segment. The average neck-shaft angle is 40 to 45 degrees (
7,
11,
12). However, more importantly, humeral neck-shaft angle demonstrates significant individual variation with a range of 30 to 55 degrees (
7,
11,
12).
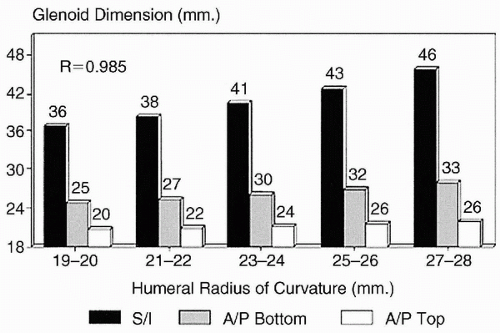
The size and shape of the articular surface of the glenoid can be defined by its linear superoinferior and anteroposterior dimensions, as well as by its radius of curvature (
7,
13). The mean superoinferior dimension of the glenoid (excluding the labrum) is approximately 39 mm (range 30 to 48 mm) (
7,
13). The anteroposterior dimension of the superior half of the glenoid is shorter than the inferior half of the glenoid, resulting in a pear-shaped appearance. The mean anteroposterior dimension of the superior half of the glenoid (excluding the labrum) is approximately 23 mm (range 18 to 30 mm), and the mean anteroposterior dimension of the inferior half of the glenoid (excluding the labrum) is approximately 29 mm (range 21 to 35 mm) (
7,
13). The ratio of the superoinferior dimension to the anteroposterior dimension of the larger, inferior half of an average glenoid is 1:0.7 (
7). The humeral head radius correlates with the size of the glenoid in both the superoinferior and anteroposterior dimensions (
Fig. 7-2) (
7).
Controversy exists about the relationship between glenoid and humeral articular radius of curvature (
7,
14,
15,
16). This controversy exists because of differences in measuring techniques, differences in sample sizes, and large individual variations in anatomy. The thickness of the articular cartilage of the glenoid increases toward the periphery of the articular surface and must be included when measuring the glenoid radius of curvature (
16). However, even when the articular cartilage is included in the measurement, the radius of curvature of the glenoid articular surface does not equal the radius of the humeral articular surface in all specimens (
7,
14,
15,
16). Iannotti and colleagues (
7) observed that, on average, the glenoid radius of curvature in the coronal plane was 2.3-mm larger than the coronal plane humeral radius of curvature in the same specimen. Soslowsky and coworkers (
16) reported a difference between humerus and glenoid radii of curvature of less than 2 mm in 88% of specimens and less than 3 mm in all specimens. Kelkar and associates (
14,
15) reported a mean humeral radius of curvature that was 2 mm less than the mean glenoid radius of curvature.
Normal glenoid version also exhibits significant individual variation (
17,
18,
19). Furthermore, the amount of measured retroversion can vary depending on the method of measurement and with the portion of the glenoid being measured. If computed tomography (CT) scanning is used to measure version, the measurement will vary with the angle of the cut—the cut must be perpendicular to the glenoid face for the version measurement to be accurate (
20). In addition, retroversion of the superior portion of the glenoid is slightly greater than the inferior portion (
21). Despite these limitations, accurate direct measurement of glenoid retroversion in cadaver specimens indicates that the glenoid is retroverted 1.23 degrees and is slightly more retroverted in white males (2.63 degrees) than in African American males (0.20 degrees) (
17).
The lateral glenohumeral offset can be defined as the perpendicular distance from the base of the coracoid process to the most lateral extent of the greater tuberosity (
7). The distance from the most lateral extent of the greater tuberosity to the lateral edge of the acromion process correlates with the lateral glenohumeral offset and is easily measured intraoperatively. Lateral glenohumeral offset is important in shoulder function because it determines capsular tension, resting length of the rotator cuff muscles, and the moment arm for the deltoid muscle. Lateral glenohumeral offset averages approximately 54 to 57 mm (range 43 to 68 mm), and the distance from the greater tuberosity to the lateral margin of the acromion process averages 17 mm (range 15 to 21 mm) (
7). Lateral glenohumeral offset and greater tuberosity to acromion distance both correlate with humeral head size and patient height (
7).
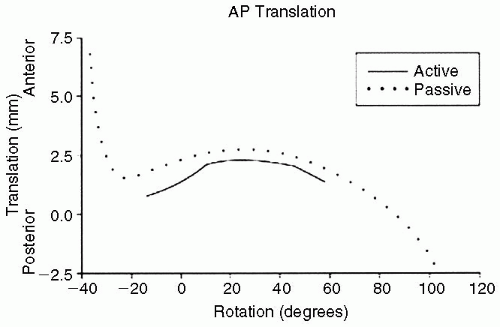
The rotator cuff and glenohumeral joint capsule function to maximize range of motion and stability in the normal shoulder. During active joint positioning within physiologic ranges of motion, the rotator cuff compresses the humeral head and dampens not only the maximum rotational motion achievable but also limits translation of the humeral head on the glenoid to 2 mm or less in most cases. The glenohumeral capsular ligaments, in their normal state, allow maximum passive range of motion and act as checkreins at the extremes of motion to prevent overrotation. In the absence of the stabilizing effect of the rotator cuff, tightness in the capsular ligaments results in greater
degrees of translation in the direction opposite the capsular tightening (
Fig. 7-3) (
22).