Abstract
Objectives
Piriformis Muscle Syndrome (PMS) is caused by sciatic nerve compression in the infrapiriformis canal. However, the pathology is poorly understood and difficult to diagnose. This study aimed to devise a clinical assessment score for PMS diagnosis and to develop a treatment strategy.
Material and methods
Two hundred and fifty patients versus 30 control patients with disco-radicular conflict, plus 30 healthy control subjects were enrolled. A range of tests was used to produce a diagnostic score for PMS and an optimum treatment strategy was proposed.
Results
A 12-point clinical scoring system was devised and a diagnosis of PMS was considered ‘probable’ when greater or equal to 8. Sensitivity and specificity of the score were 96.4% and 100%, respectively, while the positive predictive value was 100% and negative predictive value was 86.9%. Combined medication and rehabilitation treatments had a cure rate of 51.2%. Hundred and twenty-two patients (48.8%) were unresponsive to treatment and received OnabotulinumtoxinA. Visual Analogue Scale (VAS) results were ‘Very good/Good’ in 77%, ‘Average’ in 7.4% and ‘Poor’ in 15.6%. Fifteen of 19 patients unresponsive to treatment underwent surgery with ‘Very good/Good’ results in 12 cases.
Conclusions
The proposed evaluation score may facilitate PMS diagnosis and treatment standardisation. Rehabilitation has a major role associated in half of the cases with botulinum toxin injections.
Résumé
Introduction
Le syndrome du muscle piriforme (SMP) est une entité encore mal connue, responsable d’une sciatique à début fessier, véritable syndrome canalaire par compression du nerf ischiatique. Son diagnostic est clinique sachant qu’il n’existe pas de gold standard.
Méthodes
Sur la base de l’examen clinique, nous avons constitué un score diagnostique du SMP (12 items), évalué sur une série personnelle de 250 patients comparés à 30 témoins avec conflit disco-radiculaire et 30 témoins sains. À partir de cette série, une stratégie thérapeutique a été proposée, privilégiant les soins rééducatifs ciblés sur le muscle piriforme, enrichis d’alternatives thérapeutiques comme les injections de toxine botulinique ou la chirurgie dans les situations réfractaires.
Résultats
La sensibilité et la spécificité du score étaient respectivement de 96,4 % et 100 % alors que la valeur prédictive positive était de 100 % et la valeur prédictive négative de 86,9 %. Le protocole médicamenteux et rééducatif permet d’obtenir 51,2 % de guérison. Cent vingt-deux patients en échec ont bénéficié d’injections de toxine botulinique. Les résultats évalués par l’EVA étaient très bons et bons dans 94 cas (77 %), moyens dans huit cas (7,4 %) et mauvais dans 19 cas (15,6 %). Quinze des 19 patients en échec ont été pris en charge chirurgicalement avec de très bons et bons résultats dans 12 cas.
Conclusion
Le score d’évaluation proposé dans ce travail devrait permettre de faciliter le diagnostic de SMP et de standardiser son suivi. La rééducation garde une place majeure associée dans la moitié des cas au traitement par injections de toxine botulinique.
1
English version
1.1
Introduction
Piriformis Muscle Syndrome (PMS) is a neuromuscular disorder caused by the sciatic nerve becoming compressed in the infrapiriformis (sub-pyramidal) canal and occasioning sciatic-type pain, tingling, and numbness in the buttocks along the sciatic nerve pathway down to the lower thigh and into the leg . PMS is poorly understood and diagnosis is often difficult since there is no gold standard test for this condition. .
Little is known about PMS from an anatomical, biomechanical and clinical viewpoint and its diagnosis is accepted only after other causes of pain arising in the buttocks or lower limbs have been eliminated. Thus, it is a diagnosis of exclusion. Although PMS is relatively uncommon, a number of clinical signs can be identified by conducting specific tests that must be systematically performed during physical examination, particularly if the patient presents with sciatic pain without concomitant lower back pain, and if the pain varies depending upon position . As with diagnosis, there is currently no gold standard therapy for PMS and current treatment options comprise medication, rehabilitation or surgery.
To facilitate the diagnosis of PMS, this prospective study clinically assessed patients with suspected PMS and used imaging and electrophysiology techniques to try to quantify the condition in more detail. It was anticipated that, on the basis of these investigations, a clinical assessment score could then be devised that could be used in the future to diagnose PMS. Furthermore, it was hoped that a treatment strategy to optimise patient outcome could be developed.
1.2
Patients and methods
This was a prospective study conducted between June 2003 and December 2011 in the Department of Neuromuscular Examination and Diseases at the CHRU in Besançon, France.
1.2.1
Patients
1.2.1.1
Piriformis Muscle Syndrome patients
A total of 250 patients aged 18 years or above who presented with clinical symptoms suggesting PMS, which had persisted for at least 3 months, were examined and included in the study. Inclusion criteria comprised clinical features based on pain arising in the buttocks and spreading ipsilaterally to the sciatic area of the buttocks, whether or not accompanied by sensory-motor problems. Pain was required to be fluctuating throughout the course of the day and worsened by significant effort or as a result of adopting trigger positions (i.e. seated position or after standing up for a prolonged period), as well as patients experiencing pain-free periods. Exclusion criteria included signs of lumbar radicular compression, coxopathy, inflammatory or mechanical sacroiliac problems, or any inflammatory, infectious or tumour-related pelvic disease.
1.2.1.2
Control group patients
In addition, the study included two types of control group patients who were all aged 18 years or above: 30 patients who presented with symptomatic lumbar disco-radicular conflict located in regions L5 or S1 (i.e. disco-radicular controls [DRC]), and 30 healthy subjects (HS) who had no painful joints or neurological pathology.
1.2.2
Standard investigational procedures
All patients were subject to the same standard investigational procedures, which comprised the following:
- •
an interview to establish the clinical characteristics of the pain (i.e. topography, fluctuation during the day, triggers, spread), as well as the absence of pain in the lower back, and presence of sensations of distal paresthesia;
- •
a number of examinations were conducted. Clinical examination of the joints (i.e. spine, coxofemoral, sacroiliac), neurological examination to detect any sensory-motor deficits or reflex abnormalities, measurement of lower limb length using a tape measure to allow a comparison between the distance between the anterior-superior iliac crest and the medial malleolus, as well as a number of well-documented manoeuvres to test the piriformis muscle. These included the Freiberg manoeuvre for stretching the piriformis muscle , the Flexion-Adduction-Internal Rotation (FAIR) manoeuvre , the heel-contralateral knee manoeuvre (HCLK) and the Beatty test for resisted contraction . After each manoeuvre, it was noted whether or not the patient experienced triggering of spontaneous pain;
- •
standard laboratory tests consisted of a haematology, biochemistry and renal function along with coagulation and inflammatory parameters (i.e. sedimentation rate, C-reactive protein [CRP]);
- •
medical imaging comprised antero-posterior X-rays of the pelvis and hips (weight-bearing) using Lequesne’s vertical-centre-anterior margin (VCA) angle measured on the false profile view of the pelvis in order to quantify the anterior acetabular coverage of the femoral head, as well as antero-posterior and medio-lateral X-rays of the lumbar spine. A computed tomography (CT) or magnetic resonance imaging (MRI) lumbar scan was performed for all the PMS and DRC patients, and all PMS patients also underwent a pelvic MRI scan (regions T1 and T2) to investigate the presence of any morphological abnormalities of the piriformis muscle and to measure its dimensions.
- •
An electro-neuro-myography examination was conducted with electromyography (EMG) signal detection and measurements of stimulodetection patterns during the FAIR manoeuvre.
1.2.3
Piriformis Muscle Syndrome therapeutic management
Standard treatment was offered to the 250 PMS patients. First line treatment consisted of muscle relaxants and levels 1 to 2 pain relief, as well as massage-physiotherapy to allow daily self-rehabilitation and three physiotherapy sessions per week. The self-rehabilitation techniques were explained during the course of the consultation and explanatory sheets about the treatment were given to the patients ( Appendices 1 and 2 ). These techniques involved pelvic-trochanter muscle stretching and were similar to the specific manoeuvres carried out during the physical examination. Treatment by the masseur-physiotherapist was aimed at performing deep transverse massage of the affected piriformis muscle and pelvic-trochanter muscle therapy, as well as ensuring accuracy of the self-rehabilitation techniques and performing patient-specific proprioceptive pelvic-femoral exercises.
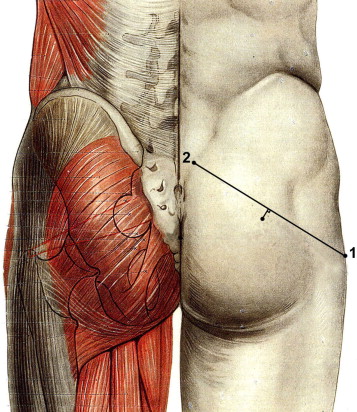
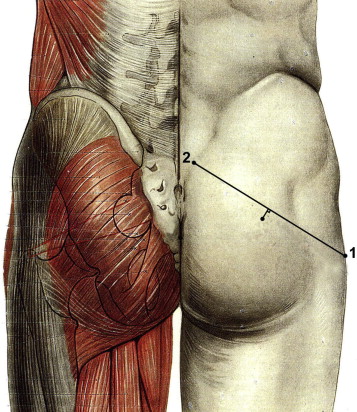
Rehabilitation was scheduled to take 6 weeks. However, those patients who reported a significant improvement in their PMS symptoms as a result of self-rehabilitation were reviewed after another 6-week period (i.e. 3 months after treatment began). Those patients who did not respond to this initial treatment continued with self-rehabilitation but were also offered one or more injections of OnabotulinumtoxinA in the piriformis muscle. Information about the risks and benefits of this treatment was given to each patient and written consent was obtained in all cases prior to injection. The piriformis muscle was identified through EMG detection using a 75 mm needle to ensure accurate injection of the target muscle, with the patient lying on his or her healthy side, with the hip and knee bent on the side of the painful limb, and with the foot wedged behind the contralateral knee. Projection of the piriformis muscle body onto the gluteal fossa was located about 1 cm below the middle of a line joining the posterior-superior iliac crest to the greater trochanter ( Figs. 1 and 2 ). Following location, the piriformis muscle was activated using active lateral rotation.
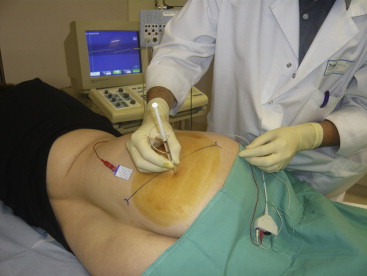
The doses of OnabotulinumtoxinA (BOTOX ® ) injected were between 50 and 100 U. Depending on the evolution, injections were renewed in accordance with a minimum of 3 months between two injections.
If no benefits were evident following three injections of OnabotulinumtoxinA, patients were informed about the potential advantages of surgery involving cutting the distal tendon of the piriformis muscle.
Regardless of the type of treatment received, buttock and sciatic pain intensity was assessed using a Visual Analogue Scale (VAS). Patients were required to rate the persistence, reduction or disappearance of painful symptoms as ‘Poor’ (no improvement), ‘Average’ (improvement of less than or equal to 50% of pain in the buttocks and/or sciatica), ‘Good’ (improvement of greater than or equal to 50% of pain in the buttocks and sciatica, or disappearance of sciatica regardless of buttock pain level) or ‘Very Good’ (disappearance of buttock and sciatica pain).
1.2.4
Statistical analysis
The results were presented as means and standard deviations for quantitative variables, and percentages for the qualitative variables. An analysis of variance (ANOVA) was used to compare the age between the three groups. The qualitative variables (i.e. proportion of men and women, positivity of the response to tests on the piriformis muscle) were compared between the three groups using the Chi 2 test. Nerve conduction delays obtained with the electromyography examination were compared between PMS and DRC patients using an unpaired Student t -test and a P -value lower than 0.05 was considered to be statistically significant. Sensitivity (Se), specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV) of the clinical assessment score for the diagnosis of PMS were also calculated.
1.3
Results
The PMS group consisted of 147 females (59%) and 103 males (41%), with an average age of 45.9 ± 11.2 years. There was no significant difference between the PMS and control groups regarding patient age and sex ( Table 1 ). PMS symptoms had been present for an average of 17 ± 11.4 months (range: 4 months to 5.5 years).
| PMS | DRC | HS | P | |
|---|---|---|---|---|
| n | 250 | 30 | 30 | |
| Sex | ||||
| M, n (%) | 103 (41) | 13 (43) | 14 (47) | NS a |
| F, n (%) | 147 (59) | 17 (57) | 16 (53) | |
| Age (years) (average ± standard deviation) | 45.9 ± 11.2 | 45.4 ± 11.9 | 43.5 ± 10.7 | NS b |
| Duration of symptoms (month) (average ± standard deviation) | 17.3 ± 11.4 | 8.8 ± 5.8 | ||
| PMS tests | ||||
| Freiberg | ||||
| Buttock, n (%) | 250 (100) | 7 (23.3) | 0 | < 0.0001 a |
| Sciatica, n (%) | 250 (100) | 7 (23.3) | 0 | < 0.0001 a |
| FAIR | ||||
| Buttock, n (%) | 250 (100) | 12 (40) | 0 | < 0.0001 a |
| Sciatica, n (%) | 250 (100) | 12 (40) | 0 | < 0.0001 a |
| TGCL | ||||
| Buttock, n (%) | 250 (100) | 12 (40) | 0 | < 0.0001 a |
| Sciatica, n (%) | 250 (100) | 12 (40) | 0 | < 0.0001 a |
| Beatty | ||||
| Buttock, n (%) | 250 (100) | 2 (6.7) | 0 | < 0.0001 a |
| Sciatica, n (%) | 250 (100) | 2 (6.7) | 0 | < 0.0001 a |
| Electro-neuro-myography examination | ||||
| Detection (acute or chronic neurogenic patterns), n (%) | 0 | 26 (86.7) | ND | |
| Stimulodetection patterns | ||||
| H-reflex (ms) (prolongation in limbs with PMS compared to the healthy side) | 0.36 ± 0.37 | 0.41 ± 0.39 | NS c | |
| FAIR manoeuvre (ms) (prolongation in limbs with PMS compared to the healthy side when tested in the FAIR position) | 0.7 ± 0.7 | 0.46 ± 0.41 | 0.06 c | |
In the PMS group, 199 patients were found to have one leg shorter than the other. For 138 of these patients, the shorter leg was on the side of the PMS (55.2%) whereas for the remaining 61 patients (24.4%), it was on the healthy side. A difference greater than 1 cm was observed for the shorter leg on the PMS side in 28 PMS patients (11.2%), and on the healthy side in 12 healthy subjects (4.8%). Eighty-three PMS patients (33.2%) had hypoesthesia or paresthesia in the superficial fibular region and 54 patients (21.6%) in the sural region. One hundred and thirteen patients (45.2%) presented with paresthesia in the whole truncal sciatic area to the foot or leg, with fluctuating symptoms during the day. Three patients (1.2%) initially presented with a partial motor deficit in the ipsilateral area of the common fibular nerve.
The DRC patients experienced radicular pain in the L5 (56.7%) or S1 (43.3%) regions and a herniated disc was identified through imaging. Six patients presented with a partial motor deficit in the corresponding area, and 21 had hypoesthesia in the corresponding radicular region.
The clinical manoeuvre tests designed to provoke pain were systematically performed in both the PMS and control groups. The manoeuvres triggered pain in 100% of the PMS patients, in 6.7 to 40% of DRC patients, and in 0% of the HS ( Table 1 ).
The combination of identifying the clinical features suggesting the presence of PMS and the standard procedures for examining the piriformis muscle allowed us to establish a clinical score for diagnosing PMS. This score comprised 12 items, each worth one point (score of 0 to 12). A diagnosis of PMS was considered to be ‘Probable’ if the score was greater or equal to 8, ‘Unlikely’ if the score was between 6 and 8, and ‘Not considered’ when the score was less than 6 ( Table 2 ). When applied to the three groups of patients (PMS, DRC and HS), the clinical assessment score was greater or equal to 8 in 241 patients (96.4%) in the PMS group versus 0 patients in the control group (after pooling both the DRC patients and HS subjects). The score was between 6 and 8 in nine PMS patients (3.6%) versus 0 in the control group, and less than 8 in 0 PMS patients versus 60 (100%) in the control group. Therefore, it was concluded that using the value of 8 was important to confirm the diagnosis of PMS. The Se and Sp of this score were calculated using this threshold value (for these calculations, the results less than 6 and those between 6 and 8 were combined). The Se and Sp of the score were 96.4% and 100%, respectively, whereas PPV was 100% and NPV 86.9% ( Table 3 ).
| Criteria | Point |
|---|---|
| Unilateral or bilateral buttock pain with fluctuating periods without pain throughout the course of the day | 1 |
| No lower back pain | 1 |
| Axial spinal palpation painless (L2 to S1) | 1 |
| Negative Lasègue’s manoeuvre | 1 |
| Seated position (often for a prolonged period) triggering buttock pain and/or sciatic pain | 1 |
| Sciatic pain with fluctuating periods without pain throughout the course of the day | 1 |
| Buttock pain next to the projection of the piriformis muscle reproduced by | |
| Stretching manoeuvres (FAIR, Freiberg, HCLK) | 1 |
| Contraction resisted manoeuvres (Beatty) | 1 |
| Palpation | 1 |
| Sciatic pain (L5, S1 or truncal sciatic area) reproduced by the extension of clinical manoeuvres (several tens of seconds) | |
| Stretching | 1 |
| Resisted contraction | 1 |
| Absence of perineal irradiation | 1 |
| Total | 12 |
| PMS | Controls | Total | |
|---|---|---|---|
| Score ≥ 8 | 241 | 0 | 241 |
| 8 < Score ≥ 6 | 9 | 0 | 9 |
| Score < 6 | 0 | 60 | 60 |
| Total | 250 | 60 | 310 |
There were no abnormalities found in any patients with respect to laboratory assessments.
The EMG detection graphs were normal for all PMS patients. In those control patients with disco-radicular conflict, 26 (86.7%) presented with signs of acute or chronic neurogenic atrophy (16 in L5, 10 in S1 regions). With respect to the stimulus-induced EMG, there was a delay of 0.36 ± 0.37 ms in the proximal nerve conduction (H-reflex) on the pathological side when compared with the healthy side in PMS patients. This delay was longer (0.41 ± 0.39 ms) in the DRC group, but it was not statistically significantly different from the PMS group ( Table 1 ).
The delay in proximal nerve conduction (H-reflex) on the pathological side compared with the healthy side, as measured with the FAIR manoeuvre, was longer in the PMS group than in the DRC group but this was not statistically significant (0.7 ± 0.7 ms versus 0.46 ± 0.41 ms [ P = 0.06]). In 46 PMS patients (18.4%), the delay was greater than 1.8 ms during the FAIR manoeuvre. In the DRC group, 11 patients (36.7%) showed a delay in conduction of 0.5 ms to 1.5 ms between both sides, with no effect from the FAIR manoeuvre. EMG was not performed in any of the healthy subjects.
Pelvic MRI imaging was performed in all PMS patients and results revealed that the size of the piriformis muscle was greater than 10% larger on the pathological side compared to the healthy side in 69 (27.6%) patients in the PMS group. Conversely, the piriformis muscle was greater than 10% bigger on the healthy side than the pathological side in 18 patients (7.2%). Pelvic MRI scans were not performed in patients assigned to either of the control groups.
After 3 months of treatment using the medication and rehabilitation protocol, 128 PMS patients (51.2%) were found to have complete disappearance of the sciatic pain (after an average period of 4 weeks) and buttock pain (after an average period of 7 weeks). These patients were allowed to continue their self-rehabilitation treatment with a clinical reassessment after 6 months. One hundred and twenty-two patients (48.8%) for whom initial treatment failed after 6 weeks were subsequently treated with OnabotulinumtoxinA injections: 51 patients (41.8%) received one single injection, 43 (35.2%) had two injections, 18 (14.8%) had three injections, nine (7.4%) had four injections and only one (0.8%) had five injections. The average interval was 18 weeks between the first and second injections (range: 12 to 31 weeks), 31 weeks between second and third injections (range: 24 to 45 weeks), 45 weeks between third and fourth injections (range: 36 to 58 weeks) and 57 weeks between fourth and the fifth injections. Pain relief according to the VAS was ‘Very good’ or ‘Good’ in 94 cases (77%), ‘Average’ in eight cases (7.4%) and ‘Poor’ in 19 cases (15.6%). No patient reported any immediate or delayed adverse event associated with OnabotulinumtoxinA treatment.
Fifteen of the 19 patients who were refractory to treatment underwent surgery involving cutting the distal tendon of the piriformis muscle behind the trochanter. Follow-up assessment 6 to 12 months after surgery found ‘Very good’ and ‘Good’ results in 12 cases, an ‘Average’ result in one case, and a ‘Poor’ result in two cases. The ‘Very Good’ and ‘Good’ scores remained consistent in all 12 patients after a post-treatment period of between 1 to 4 years.
1.4
Discussion
PMS is a neuromuscular disorder occasioned by the sciatic nerve becoming compressed in the infrapiriformis canal causing sciatic-type pain, tingling and numbness in the buttocks along the sciatic nerve pathway down to the lower thigh and into the leg. The aetiology of this compression is usually muscular, involving the piriformis muscle due to its location in the gluteal fossa. As the muscle contracts, it is thought to cause buttock pain which spreads ipsilaterally to the sciatic area of the buttocks. Although the classification of PMS remains controversial, it is, nevertheless, one of the rare causes of non-spinal sciatic pain. A difference in leg length is sometimes proposed as a contributing factor to PMS as stress may be exaggerated on the side of the shorter leg . However, this hypothesis was not validated in our study.
The conflicting descriptions of PMS in the literature are probably largely due to inaccurate patient selection criteria and to the absence of validated clinical criteria . For the purposes of maintaining consistency with respect to the physiopathology of PMS, in this study patient inclusion criteria focused on those symptoms, which combined pain in the piriformis muscle and compression of the sciatic nerve. Symptoms were required to fluctuate during the day and manifest themselves in different ways depending on changes in position. The influence of the position was supported by both anatomical and radiological studies. The piriformis muscle is stretched still further when the patient moves from a standing position into a seated position, and when in the position of sitting with crossed legs. In these cases, the configuration of the infrapiriformis canal may be indirectly changed .
There are specific clinical manoeuvres that are likely to reproduce the pain caused by PMS. The results of this study validate the four clinical manoeuvres that we used as being relevant for the diagnosis of PMS. These manoeuvres were found to be positive in 100% of PMS patients compared with between 6.7 to 40% of the control patients with disc-related sciatica, depending on the particular manoeuvre under consideration. It is important that these manoeuvres are performed over several tens of seconds (up to 1 minute) in order to trigger buttock pain that subsequently spreads to the sciatic area.
The EMG signal detection examination was normal in the PMS patients, whereas it was found to be impaired in the DRC group. The absence of any neurogenic signs in the L5 and S1 regions in the PMS patients reinforces the theory of the importance of positioning for eliciting symptoms. In PMS patients, EMG stimulus detection was found to be useful during the FAIR test manoeuvre, thus further reinforcing the notion of the positional nature of this syndrome . A future prospective study investigating the advantages of prolonging the FAIR test manoeuvre to sensitise the delay in conduction could be useful.
The imaging data relating to the piriformis muscle made very little contribution to our understanding of PMS. No signal abnormalities either from the piriformis muscle or the sciatic nerve were found, and there was no evidence of any muscular hypertrophy, contrary to some findings in the medical literature .
As discussed earlier, there is currently no gold standard test for this pathology. However, the standardised method of examination of the 250 PMS patients in this study allowed us to propose a score for diagnosing PMS. Our findings showed the Se, Sp and PPV of this score to be very high and, once validated, this tool could be used to include patients in future PMS studies and it should also allow accurate PMS monitoring over time and to assess the effects of treatment.
In the 250 PMS patients enrolled in this study, rehabilitation treatment was effective in more than half of the cases. However, it is important to both educate and supervise patients closely to ensure that self-rehabilitation methods are effective and issuing explanatory leaflets to patients was found to be a useful tool in optimising this treatment.
In cases where rehabilitation treatments did not confer benefits, patients were offered OnabotulinumtoxinA injections . OnabotulinumtoxinA blocks presynaptic conduction, thus inhibiting cholinergic mediation, which prevents the injected muscle from contracting, thereby creating temporary paresis. Intramuscular injections of OnabotulinumtoxinA are used to reduce muscle hyperactivity, and are particularly effective for treatment of focal spasticity . In PMS, OnabotulinumtoxinA reduces piriformis muscle contraction and thus relieves sciatic pain.
All the patients who underwent OnabotulinumtoxinA injections received between 50 to 100 U, depending on the estimated piriformis muscle volume. The muscle was injected at two sites because the structure of the piriformis muscle usually comprises two juxtaposed spindle-shaped muscular bundles. As the piriformis muscle is situated in a deep location, it was felt necessary to use a localisation technique prior to the injection and, in accordance with many other investigators, an EMG-guided approach was taken. This has the advantage of being simple to carry out and provides an accurate method of intramuscular localisation .
In this study, the results of the OnabotulinumtoxinA injection were ‘Very good’ or ‘Good’ in 77% of cases, however, this was an open study, without any comparison group. Nevertheless, our results were consistent with the literature where there are reports from small-scale studies that show the effectiveness of OnabotulinumtoxinA injections in PMS treatment .
The role of surgery and the nature of the surgical procedure in PMS remain controversial , in particular with respect to neurolysis. In our experience, surgery involving simple distal disinsertion of the piriformis muscle proved effective in most cases, with 12 out of 15 patients reporting ‘Very good’ or ‘Good’ results even after several years of follow-up.
1.5
Conclusion
The problems caused by PMS are as a result of its physiopathology, which is currently poorly understood. The clinical symptoms reported by patients and triggered by specific physical tests suggest a spasm-type pain in which the sciatic nerve becomes compressed in the infrapiriformis canal causing sciatic-type pain. It is hoped that the scoring system devised in this study will facilitate the diagnosis of PMS and standardise its follow-up for the future.
Treatments acting on the piriformis muscle itself improve symptoms, especially the use of targeted stretching manoeuvres, particularly when combined with OnabotulinumtoxinA injections. However, it is important to conduct a controlled study in the future to assess the effectiveness of these injections and evaluate their role in the treatment of PMS. Surgical treatment by cutting the distal tendon of the piriformis muscle could also be used in some patients who are refractory to other treatment. The effectiveness of all these treatments confirms the muscular physiopathological hypothesis of PMS.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Appendix A
Supplementary data
Supplementary data (Rehabilitation treatment protocol for PMS) associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.rehab.2013.04.003 .
2
Version française
2.1
Introduction
Le syndrome du muscle piriforme (SMP) est une entité encore mal connue et son diagnostic sujet à controverse . Il est considéré comme un équivalent de syndrome canalaire par compression du nerf ischiatique dans le canal infrapiriforme et s’exprime sous forme d’une douleur à type de sciatique à départ fessier . Cette pathologie est mal connue sur le plan anatomique, biomécanique et clinique et son diagnostic est retenu après avoir éliminé d’autres causes de douleurs fessières et/ou du membre inférieur. Il s’agit donc d’un diagnostic d’exclusion. Sa fréquence, probablement faible, ne doit pas faire oublier un certain nombre de signes cliniques à rechercher systématiquement à l’examen physique par des tests spécifiques , devant un tableau de sciatalgie sans lombalgie, ce d’autant que l’atteinte douloureuse est fluctuante et positionnelle. Il n’existe pas actuellement à l’examen clinique de test qui soit une référence. Par ailleurs, la prise en charge thérapeutique du SMP n’est pas standardisée. Elle fait appel à des traitements médicamenteux, à la rééducation ou à la chirurgie.
Pour faciliter le diagnostic de SMP, nous avons réalisé un travail prospectif visant à évaluer cliniquement, en imagerie et en électrophysiologie une série de patients suspects de SMP. Ce travail nous a permis d’élaborer un score d’évaluation clinique permettant de retenir le diagnostic de SMP. Sur le plan thérapeutique à partir de cette série, nous avons élaboré une stratégie de prise en charge, en privilégiant d’abord les soins rééducatifs ciblés sur le muscle piriforme, puis en proposant des alternatives comme les injections de toxine botulinique ou le recours à la chirurgie dans les situations réfractaires.
2.2
Patients et méthodes
Il s’agit d’un travail prospectif mené de juin 2003 à décembre 2011 au sein du service d’explorations et de pathologies neuro-musculaires du CHRU de Besançon.
2.2.1
Patients
2.2.1.1
Patients souffrant de syndrome du muscle piriforme
Deux cent cinquante sujets d’âge supérieur ou égal à 18 ans présentant une symptomatologie clinique évocatrice de SMP évoluant depuis au moins trois mois ont été examinés et inclus dans la série. Les critères d’inclusion étaient cliniques, reposant sur une douleur fessière à irradiation homolatérale dans un territoire sciatique débutant à la fesse accompagnée ou non de troubles sensitivo-moteurs et dont l’évolution fluctuante était favorisée par des efforts importants ou des positions déclenchantes (position assise ou orthostatisme prolongé). Les patients devaient présenter une association obligatoire d’une fessalgie et d’une sciatique à début fessier, toutes deux fluctuantes au cours d’une même journée avec souvent des périodes non douloureuses. Les critères d’exclusion correspondaient à des signes de compression radiculaire lombaire, une coxopathie, une souffrance sacro-iliaque inflammatoire ou mécanique ou une pathologie pelvienne inflammatoire, infectieuse ou tumorale.
2.2.1.2
Témoins
Deux groupes de sujets étaient analysés : 30 sujets présentant un conflit disco-radiculaire symptomatique (témoins disco-radiculaires [TDR]) lombaire de topographie L5 ou S1 documenté et 30 témoins sains (TS), sans aucune pathologie douloureuse articulaire ou neurologique, d’un âge supérieur ou égal à 18 ans.
2.2.2
Procédure standardisée d’exploration
Pour chaque sujet exploré (SMP, TDR et TS) étaient réalisés :
- •
un interrogatoire précisant les caractéristiques cliniques de la douleur (topographie, évolution fluctuante dans la journée, déclenchement, les irradiations), l’absence de lombalgies, la notion de paresthésies distales ;
- •
un examen clinique articulaire (rachidien, coxofémoral, sacroiliaque), neurologique (recherche d’un déficit sensitivomoteur ou des anomalies des réflexes), une mensuration des membres inférieurs (mesure comparative au mètre ruban de la distance épine iliaque antéro-supérieure/malléole médiale) ainsi que les manœuvres sollicitant le muscle piriforme : manœuvre d’étirement de Freiberg , manœuvre FAIR (Flexion-Adduction-Internal Rotation) , manœuvre talon genou controlatéral (TGCL) et manœuvre de contraction résistée de Beatty . Ces manœuvres sont décrites dans la littérature et la manœuvre TGCL s’appuie sur notre expérience personnelle. Pour chaque manœuvre était noté le déclenchement ou non de la douleur ressentie spontanément par le patient ;
- •
un bilan biologique standard comportant hémogramme, biochimie et fonction rénale, coagulation et les paramètres inflammatoires (vitesse de sédimentation, CRP) ;
- •
des examens d’imagerie : il s‘agissait de radiographies en charge du bassin et des hanches de face avec un faux profil de Lequesne; de radiographies du rachis lombaire de face et profil. Un scanner lombaire ou une IRM lombaire étaient effectués pour tous les patients SMP et les TDR. Une IRM du bassin (séquences T1 et T2) recherchait chez tous les patients SMP des anomalies morphologiques du muscle piriforme et permettait d‘obtenir les dimensions de ce muscle ;
- •
un examen électroneuromyographique avec une étude en électromyographie (EMG) de détection et des mesures en stimulodétection sensibilisées par la manœuvre FAIR.
2.2.3
Prise en charge thérapeutique du syndrome du muscle piriforme
Pour les 250 patients SMP, une prise en charge thérapeutique standardisée a été proposée.
Le premier traitement comportait des myorelaxants et antalgiques de niveau 1 à 2, ainsi qu’une prescription de masso-kinésithérapie proposant une auto-rééducation quotidienne et trois séances par semaine au cabinet du kinésithérapeute. Les techniques d’auto-rééducation étaient expliquées durant la consultation et des fiches explicatives de rééducation étaient remises au patient ( Annexes 1 et 2 ). Ces techniques correspondaient à des mises en situation d’étirements des muscles pelvi-trochantériens, postures proches des manœuvres spécifiques réalisées lors de l’examen physique. La prise en charge par le masseur-kinésithérapeute avait pour buts la réalisation de massages transverses profonds du muscle piriforme souffrant, un travail myotensif guidé des muscles pelvi-trochantériens, un contrôle de la justesse des techniques d’auto-rééducation et un travail « proprioceptif pelvi-fémoral ». Ce travail rééducatif était proposé sur une période de six semaines. Les patients décrivant une amélioration significative par l’auto-rééducation étaient revus après un délai de six semaines supplémentaires (soit trois mois après le début de la prise en charge).
Pour les patients ne répondant pas à ce premier traitement, il leur était proposé, tout en poursuivant l’auto-rééducation une ou plusieurs injections de toxine botulinique dans le muscle piriforme, avec une aiguille de 75 mms permettant le repérage électrophysiologique et l’injection. Une information sur l’intérêt et les risques de ce traitement était donnée et un consentement écrit obtenu pour chaque patient. Les doses injectées étaient comprises entre 50 et 100 U de toxine botulinique sérotype A (Botox ® ). Le muscle piriforme était repéré grâce à l’EMG de détection, chez un patient en décubitus latéral sur le côté sain, hanche et genou fléchis du côté du membre douloureux, le pied étant bloqué derrière le genou controlatéral. La projection du corps musculaire du muscle piriforme dans la fosse glutéale était localisée environ 1 cm sous le milieu d’une ligne joignant l’épine iliaque postéro-supérieure au grand trochanter ( Fig. 1 et 2 ). L’activation du muscle piriforme se faisait par une rotation latérale active. En fonction de l’évolution, les injections étaient renouvelées, en respectant un délai minimum de trois mois entre deux injections.