Perthes Disease
B. David Horn
David A. Spiegel
CLINICAL PRESENTATION
Perthes disease may be best described as idiopathic osteonecrosis of the femoral head in children.1 While the etiology remains unknown, the pathophysiology is believed to involve an interruption to the blood supply (segmental or total) of the capital femoral epiphysis.1 The disease is most common in boys from 4 to 8 years of age and is bilateral in 10% to 12% of cases. Up to 3% of patients have had a history of transient synovitis of the hip, and approximately 90% will have a decrease in bone age (mean of 21 months). Short stature is exhibited in 90% of patients, and approximately one-third of affected patients also have attention deficit hyperactivity disorder. While several reports have implicated thrombophilia as a cause of the disorder, more recent studies find no evidence of a heritable coagulopathy. The condition has also been associated with exposure to secondhand smoke and with a defect in type II collagen.1 Although there is a positive family history in 1% to 20% of patients, there is no evidence that Perthes disease is inherited.
Patients present with the insidious onset of a limp, and pain is often experienced in the groin, thigh, or knee. The pain usually occurs with activity and is relieved by rest. Fever is typically absent.
CLINICAL POINTS
The disease is most common in young boys (4-8 years of age).
Short stature is a characteristic of most patients.
Rest relieves the pain that occurs with activity.
PHYSICAL FINDINGS
On physical examination, there is a decrease in range of abduction and inward rotation of the hip, and there may be discomfort or guarding at the extremes of rotation. Muscle spasm may also be observed, and atrophy of the gluteal muscles is a common physical finding. During ambulation, patients tend to shift the body weight over the involved hip during stance phase (Trendelenburg gait). The Trendelenburg test should also be performed. The patient is observed from behind and is asked to lift the nonaffected leg off the ground. Normally, the gluteal muscles on the stance side will contract and maintain the contralateral hemipelvis in an elevated position. In the presence of gluteal weakness (or pain), the contralateral hemipelvis will drop down. Patients should be tested for 10 seconds.
STUDIES (LABS, X-RAYS)
Laboratory
Laboratory studies are commonly performed in children who present with a limp, including a complete blood count with a differential, erythrocyte sedimentation rate, and C-reactive protein. Additional studies such as Lyme titer, RH factor, antistreptolysin O titer, and antinuclear antibody may also be considered.
Imaging
Plain films (anteroposterior [AP] and frog-leg lateral views) are usually all that is required to make the diagnosis of Perthes disease, although additional studies may be necessary when other diagnoses are suspected. Plain radiographs help to determine the extent of physeal involvement and are used to follow patients through the course of their disease.
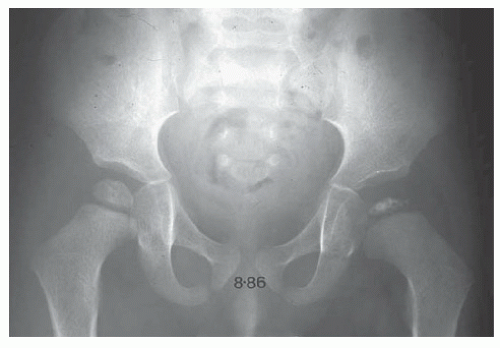
The radiographic findings evolve in four stages over 2 to 5 years, as described by Waldenstrom.1 The earliest findings (initial stage) include a decrease in the size of the ossific nucleus relative to the contralateral side, relative radiodensity
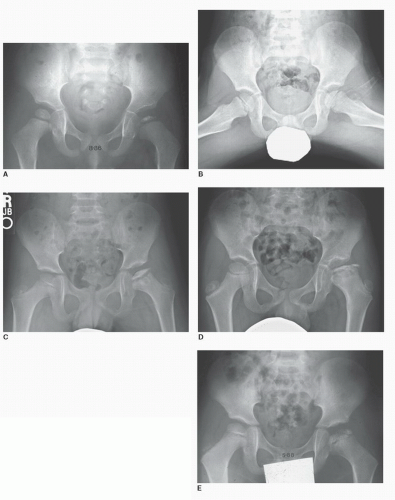
of the affected epiphysis, and an increase in the medial joint space (Fig. 57-1A). Next is the fragmentation stage, in which there are areas of both radiolucency and radiodensity within the capital femoral epiphysis, generally during the first 6 to 9 months (Fig. 57-1B-E). A subchondral fracture (“crescent sign”) may be identified on the frog lateral. Following collapse of the capital femoral epiphysis, the disease progresses to the reossification stage, in which healing of the
femoral head occurs. Areas of increased radiodensity are seen in regions that were radiolucent, along with changes in the shape of the femoral head, neck, and acetabulum. In the healed stage, permanent changes in morphology are seen. In cases of bilateral disease, each hip is typically at a different stage of the disease.
of the affected epiphysis, and an increase in the medial joint space (Fig. 57-1A). Next is the fragmentation stage, in which there are areas of both radiolucency and radiodensity within the capital femoral epiphysis, generally during the first 6 to 9 months (Fig. 57-1B-E). A subchondral fracture (“crescent sign”) may be identified on the frog lateral. Following collapse of the capital femoral epiphysis, the disease progresses to the reossification stage, in which healing of the
femoral head occurs. Areas of increased radiodensity are seen in regions that were radiolucent, along with changes in the shape of the femoral head, neck, and acetabulum. In the healed stage, permanent changes in morphology are seen. In cases of bilateral disease, each hip is typically at a different stage of the disease.
Several patterns of residual deformity may be observed. The radiographic outcome depends on the degree of involvement of the capital femoral epiphysis and the presence of physeal involvement. Coxa magna (enlarged femoral head) is common, and there may be flattening of the femoral head. Disturbance of proximal femoral physeal growth may result in shortening (coxa breva) and widening of the femoral neck. The growth cartilage of the greater trochanter is spared, resulting in a relative overgrowth of the trochanter in relation to the femoral head and may disturb hip abductor mechanics, resulting in a limp. Asymmetric physeal arrest may result in valgus angulation of the proximal femur and a more horizontal physis. Osteochondral lesions may also be seen, especially in older patients.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree