Periprosthetic Femur Fractures
Richard F. Kyle
Jonathan C. Haas
Patrick Yoon
Periprosthetic Fracture Following Total Hip Arthroplasty
Indications/Contraindications
Periprosthetic fractures around total joint replacements are becoming an increasingly common problem encountered by orthopedic surgeons. Not only is the number of total joint replacements performed increasing annually, but the expected longevity of patients who undergo these procedures is also increasing. These fractures may occur intraoperatively or postoperatively. The incidence of periprosthetic hip fractures is generally higher in uncemented (4.1% to 27.8%) than in cemented (<3%) prosthesis. Risk factors for intraoperative fractures include rheumatoid arthritis, cementless arthroplasty, metabolic bone disease, complex deformity, and revision surgery. Risk factors for postoperative fractures include weakened bone secondary to osteoporosis, stress risers, empty screw holes, eccentric reaming, cortical perforations, stem tip protrusion, loose implants, and osteolysis.
While periprosthetic fractures may occur on either the acetabular or femoral side, most of the literature discusses fractures on the femoral side of a total hip arthroplasty (THA) or around the femoral side of a total knee arthroplasty (TKA). In the first section of the chapter, we describe techniques for repairing periprosthetic fractures adjacent to the femoral component of a total hip arthroplasty. We address fractures associated with TKA in the second part of this chapter.
Several classification schemes are useful in guiding treatment of THA. Johannson classified periprosthetic femur fractures into type I (proximal to the stem tip), type II (around the tip), and type III (distal to the tip) categories. In a primary cementless implant the majority of intraoperative fractures are type I with the minority being types II or III. Duncan and Masri’s classification roughly correlates with Johannson’s classification: type A (peritrochanteric), B (tip), and C (distal) fractures. The incidence of these is reported to be 4%, 87%, and 9% respectively for types A, B, and C. Type A fractures are subdivided into A(G) (greater trochanteric) and A(L) (lesser trochanteric) fractures, which are generally treated
nonoperatively. B fractures are divided among B1 (stable prosthesis, 18%), B2 (loose prosthesis requiring stem revision, 45%), and B3 (marked osteopenia, 37%). Types I and A are the most common intraoperative fracture and can be treated with cerclage wiring. Types II and B generally require a plate, allograft, and cables. Types III and C can generally be treated without regard to the prosthesis if they are sufficiently distal.
nonoperatively. B fractures are divided among B1 (stable prosthesis, 18%), B2 (loose prosthesis requiring stem revision, 45%), and B3 (marked osteopenia, 37%). Types I and A are the most common intraoperative fracture and can be treated with cerclage wiring. Types II and B generally require a plate, allograft, and cables. Types III and C can generally be treated without regard to the prosthesis if they are sufficiently distal.
Prevention of periprosthetic fractures includes correcting risk factors that are reversible; avoiding intraoperative techniques that create stress risers, such as eccentric reaming; performing routine surveillance x-rays to check for osteolysis; and revising prostheses when progressive osteolysis is presented. It also calls for aggressive treatment for patients with osteoporosis.
Our main goal is to restore the patient to prefracture level of function. In general, surgical treatment is preferred except for the high-risk surgical patient. Although nonoperative treatment is associated with high union rates, it exposes the patient to risks associated with prolonged bed rest and greater rates of malunion. Nonoperative treatment may be appropriate for the stable type I or A fracture noted postoperatively or perhaps for a minimally displaced type II or III fracture.
The vast majority of displaced fractures are treated operatively. Treatment options for an intact prosthesis include cerclage wiring in high fractures and the use of plating and cortical struts in low fractures. Occasionally, only wires or cables are used for a very long spiral fracture that is minimally displaced. In general, however, some combination of cables, plates, and strut allografts is used. We have found that either two struts or a combination of one plate and one strut have proven to be the strongest constructs tested in torsion and bending. Any defects or stress risers created (tip of stem, cortical perforation, etc.) should be bypassed by a strut or plate by at least two cortical diameters because this strategy restores 84% of the original strength of the bone. With loose implants, treatment options include removal of the implant while maintaining as much bone stock as possible. A loose implant should then be revised with a longer stem and cortical strut grafts.
Acetabular fractures during or after THA are uncommon. Lewallen et al classified these into type I (stable cup) and type II (unstable cup). Type I fractures may be treated nonoperatively with good healing rates through use of a brace or spica cast. Type II fractures should be treated with cup revision.
Preoperative Planning
Adequate radiographs are needed to identify the extent of the fracture, stability of the prosthesis, and the quality of the bone. The standard radiographs include a low anteroposterior (AP) pelvis x-ray centered about the pubis, as well as an AP and frog or cross-table lateral of the affected limb. If acetabular osteolysis is seen or suspected, then Judet films should be obtained to assess the area and extent of bony peri-acetabular deficiency. In some patients, additional information can be gained from scanograms to assess leg lengths. Identification of prosthesis type is necessary if special femoral-extraction devices may be needed. The preoperative assessment also includes range-of-motion and neurovascular statuses so that any preoperative deficiencies and the patient’s leg lengths can be documented. If a plate is to be used, we template the fracture and determine the length of side plate needed to bridge the fracture site adequately; we will always err on using a plate that is too long. Occasionally a plate must span the entire length of the femur.
Previous operative reports are important so the surgeon can identify the surgical approach used and the type, size, and modularity of the prosthesis implanted. Preoperative templating is necessary to assess the length and diameter of the prosthesis when revision arthroplasty is needed. As a general rule, the surgeon should make sure that all stress risers, such as cortical windows and holes in the diaphysis, are bypassed with a stem that is at least two times the shaft diameter. Areas of transition between stem tips and plates or stem tips and stem tips should be avoided. Cortical strut grafts over holes, windows, fractures, and in areas of transition are beneficial. Most nonmodular stems are less than 265 cm, so the use of longer modular stems may be needed. The stem diameter should be large enough so the fit helps splint the fracture and allows an adequate surface for bone ingrowth and
good rotational control of the prosthesis. Preoperative templating is crucial because it allows the surgeon to anticipate the need for all implants.
good rotational control of the prosthesis. Preoperative templating is crucial because it allows the surgeon to anticipate the need for all implants.
If infection is suspected, a complete blood count (CBC), differential C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) should be obtained. If any of these parameters are elevated, then a hip aspiration under fluoroscopic guidance should be preformed. Because revision surgery generally entails a relatively high blood loss, patients should be typed and cross matched for blood replacement in case it is needed. We do not routinely have patients predonate blood and frequently use a cell saver.
Surgery
Patient Positioning
Because surgery may take several hours, a Foley catheter is routinely inserted, particularly if an epidural anesthetic is administered. The patient is placed in the lateral decubitus position, affected side up, on a radiolucent table with the affected hip and leg draped free. A lateral positioner is used, and all bony prominences are padded. The wider posterior portion of the holder abuts the posterior iliac crest, and the narrow anterior portion should come up directly against the anterior–superior iliac spine. The common error is to place the anterior positioner too distal, impeding full-hip flexion. The knees and the feet are lined up to use as a gauge for leg lengths. The entire lower extremity, hip, and pelvis are prepped and draped.
Technique
Nondisplaced Intraoperative Proximal Femur (Type I)
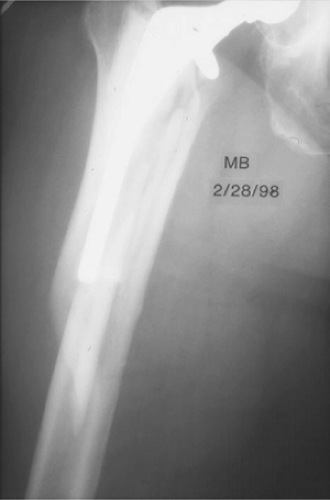
As prophylaxis against propagating a crack in the proximal femur, a Verbrugge clamp is placed around the proximal femur just prior to surgeon impact on an uncemented femoral stem. Should a fracture occur, it will likely be nondisplaced, and evidence has shown that it is unlikely to influence the long-term outcome of the joint replacement. When the fracture is recognized, one or more cerclage wires or cables are sufficient. If it is displaced, then it is reduced and
cerclage wires are used (Fig. 45.1).
cerclage wires are used (Fig. 45.1).
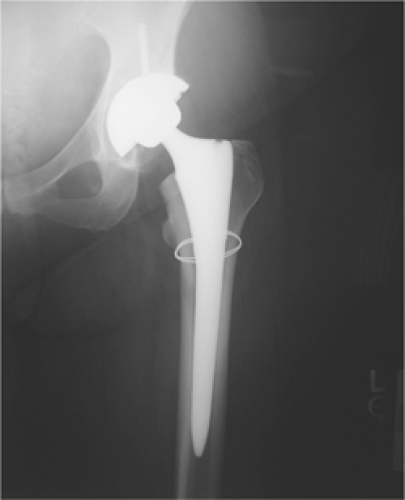
Fracture at the Tip of the Femoral Component (Type II with Loose Prosthesis)
If the prosthesis is loose in a femoral tip fracture (Fig. 45.2), a long-stem revision with allograft struts is used to bridge the tip of the stem and fracture site. Cortical perforations are used as needed to extract the old prosthesis.
The surgeon begins with the proximal part of the incision to minimize blood loss and define the plane between the iliotibial band and the vastus lateralis. This incision can be difficult to make due to scarring, but developing this layer will allow easier placement of retractors and facilitate closure. Electrocautery is used while the surgeon works to stay on bone until the neck of the prosthesis is identified.
Once joint fluid is encountered, it is sent off for culture and gram stain. Multiple fluid and tissue cultures are obtained and sent to the laboratory with every hip revision surgery. Infection can be a cause of prosthesis loosening and subsequent fracture.
Usually abundant pseudocapsule is excised until the acetabulum can be adequately visualized. The proximal femur is skeletonized to allow sufficient mobility for stem extraction and adequate acetabular exposure. A saw or osteotome is used to remove any bone from the greater trochanter that overhangs the lateral aspect of the prosthesis. Failing to remove the bone will result in a greater trochanteric fracture when the stem is removed.
Once the stem is removed, the femoral fracture is addressed. The goal is to reduce and stabilize the fracture. The midlateral incision should be extended distally past the fracture. The vastus split is facilitated by blunt dissection in line with its fibers, and care is taken to clamp and cauterize the perforators. The fracture is identified and reduced with minimal stripping, and multiple Verbrugge clamps are carefully placed. The femur may then be reamed and broached, and the femoral component tried at this time. The legs are assessed for length and stability.
Prior to dislocating the hip, the surgeon first places a suture in the skin just superior to the incision and a second marker stitch in the greater trochanter. Second, both knees and feet are aligned and checked for any length discrepancies. Third, with the trial component in place, the knee is flexed so the surgeon can check for quadriceps tightness. Occasionally, fluoroscopy is used to check the fracture reduction, the stem for length, and the percentage of canal fill. One or two cortical struts should be contoured to the femur through use of a
high-speed burr. The goal is to minimize the concavity of the graft so the fit between the graft and the femur is intimate. Wires or cables are passed first before the struts to facilitate ease of passing the wires. With the femoral trial still in place, a cortical strut graft is applied laterally to the anterior on the femur, and if needed, it is applied medially to the anterior on the femur; the graft location is dependent on the fracture pattern and the quality of bone. The allograft struts are secured with multiple wires or cables spaced at least 3.0 cm apart (Fig. 45.3). The strut should extend distal to the fracture at least 2.5 times the shaft diameter to enhance fixation and prevent propagation of the fracture. Securing the cortical struts will help prevent fracture displacement and propagation with seating of the final implant. In most cases, it is best to reduce the fracture and use the long-stem implant like an intramedullary nail to fix the fracture. Bone struts are applied after implanting to reinforce fracture fixation and rebuild bone stock. We prefer Luque wires because they are relatively inexpensive and will not cut through the bone like cables. The twist of the Luque wire should be directed posteriorly where its prominence cannot be felt. If there are risk factors for delayed union or nonunion, bank bone graft is placed at the fracture site. The final prosthesis is then seated. (Fig. 45.4), and leg lengths and stability are once again checked.
high-speed burr. The goal is to minimize the concavity of the graft so the fit between the graft and the femur is intimate. Wires or cables are passed first before the struts to facilitate ease of passing the wires. With the femoral trial still in place, a cortical strut graft is applied laterally to the anterior on the femur, and if needed, it is applied medially to the anterior on the femur; the graft location is dependent on the fracture pattern and the quality of bone. The allograft struts are secured with multiple wires or cables spaced at least 3.0 cm apart (Fig. 45.3). The strut should extend distal to the fracture at least 2.5 times the shaft diameter to enhance fixation and prevent propagation of the fracture. Securing the cortical struts will help prevent fracture displacement and propagation with seating of the final implant. In most cases, it is best to reduce the fracture and use the long-stem implant like an intramedullary nail to fix the fracture. Bone struts are applied after implanting to reinforce fracture fixation and rebuild bone stock. We prefer Luque wires because they are relatively inexpensive and will not cut through the bone like cables. The twist of the Luque wire should be directed posteriorly where its prominence cannot be felt. If there are risk factors for delayed union or nonunion, bank bone graft is placed at the fracture site. The final prosthesis is then seated. (Fig. 45.4), and leg lengths and stability are once again checked.
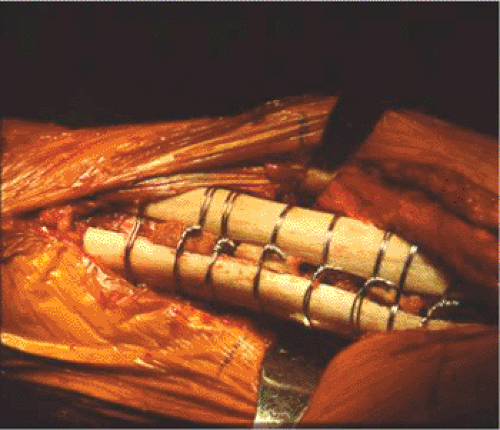 Figure 45.3. Allograft cortical struts around the proximal femur secured with multiple Luque wires. |