In this article, an overview is provided of pediatric spinal cord injury, organized by effects of this injury on various organ systems. Specific management differences between children and adults with spinal cord injury are highlighted. A detailed management approach is offered for particularly complex topics, such as spasticity and upper extremity reconstruction.
Key points
- •
Congenital and acquired pediatric spinal cord injuries (SCI) pose unique management challenges because of the dynamic nature of cognitive and physical development in the growing child and the impact of the SCI on this complex process.
- •
Care for children and adolescents with SCI should be developmentally based, using appropriate strategies to facilitate adjustment and maximize independence across the spectrum of physical and emotional maturity levels.
- •
The goal of SCI rehabilitation for children and adolescents is to maximize function and independence and to prepare them for a successful transition to adulthood.
- •
Depending on age at injury, the acquisition of certain skills (eg, bowel and bladder continence) may not be rehabilitation, but rather habilitation, because they may have never achieved this skill previously.
Video of tendon transfer surgery accompanies this article at http://www.pmr.theclinics.com/
Introduction
Congenital and acquired pediatric spinal cord injuries (SCI) pose unique management challenges because of the dynamic nature of cognitive and physical development in the growing child and the impact of the SCI on this complex process. In this review, an overview is provided of pediatric SCI rehabilitation management, highlighting how it differs from adult SCI management. Care for children and adolescents with SCI should be developmentally based, using appropriate strategies to facilitate adjustment and maximize independence across the spectrum of physical and emotional maturity levels. Emphasis should be placed on a family-centered approach to patient care, given the central role of parents and family in a child or adolescent’s life. Adolescents may not identify with the traditional pediatric model of care but are often not appropriate for an adult rehabilitation unit, which creates additional challenges when choosing the optimal therapeutic environment. Ideally, children and adolescents would receive care in a setting with age-appropriate peers having similar disabilities. However, the low incidence of pediatric SCI in the United States and the multitude of regional pediatric rehabilitation centers make the reality of a dedicated SCI unit for this population difficult to maintain and may require patients to receive care a great distance from their home and extended peer and community support network. The goal of SCI rehabilitation for children and adolescents is to maximize function and independence and to prepare them for a successful transition to adulthood. Depending on age at injury, the acquisition of certain skills (eg, bowel and bladder continence) may not be rehabilitation, but rather habilitation, because they may have never achieved this skill previously. For this reason, goal setting in therapy must be developmentally appropriate and may require a staged approach, whereby advanced level skills for a young child are deferred to a later date.
Introduction
Congenital and acquired pediatric spinal cord injuries (SCI) pose unique management challenges because of the dynamic nature of cognitive and physical development in the growing child and the impact of the SCI on this complex process. In this review, an overview is provided of pediatric SCI rehabilitation management, highlighting how it differs from adult SCI management. Care for children and adolescents with SCI should be developmentally based, using appropriate strategies to facilitate adjustment and maximize independence across the spectrum of physical and emotional maturity levels. Emphasis should be placed on a family-centered approach to patient care, given the central role of parents and family in a child or adolescent’s life. Adolescents may not identify with the traditional pediatric model of care but are often not appropriate for an adult rehabilitation unit, which creates additional challenges when choosing the optimal therapeutic environment. Ideally, children and adolescents would receive care in a setting with age-appropriate peers having similar disabilities. However, the low incidence of pediatric SCI in the United States and the multitude of regional pediatric rehabilitation centers make the reality of a dedicated SCI unit for this population difficult to maintain and may require patients to receive care a great distance from their home and extended peer and community support network. The goal of SCI rehabilitation for children and adolescents is to maximize function and independence and to prepare them for a successful transition to adulthood. Depending on age at injury, the acquisition of certain skills (eg, bowel and bladder continence) may not be rehabilitation, but rather habilitation, because they may have never achieved this skill previously. For this reason, goal setting in therapy must be developmentally appropriate and may require a staged approach, whereby advanced level skills for a young child are deferred to a later date.
Epidemiology
A population-based database for SCI in the United States does not exist, and, thus, the epidemiology of pediatric and adult SCI has been extrapolated from the National SCI Statistical Center database and the Shriners Hospital SCI database. Using these data sets, the annual incidence of SCI in the United States is estimated to be between 25 and 59 new cases per million. Assuming an average of 40 cases/million, this incidence means approximately 12,400 new SCI as estimated for the year 2010. Of these new injuries, approximately 3% to 5% of the SCIs are in individuals younger than 15 years. In adolescents, as in adults, males outnumber females by a ratio of 4:1. The preponderance of males over females with SCI decreases with earlier age at onset, and by 3 years of age, the number of females with SCI equals that of male.
The neurologic level of injury and degree of completeness of the SCI varies with age. For example, infants and younger children with tetraplegia are more likely to have upper cervical injuries (C1-3) compared with adolescents (C4-8), because of a disproportionately large head and underdeveloped neck musculature. The International Standards for Neurological Classification of SCI (ISCSCI) is the means by which the neurologic level of injury and degree of completeness is determined. In children, the reliability of the ISCSCI motor and sensory scores is good for children 5 years of age and older. However, the anorectal examination, which is a frequent determinant of completeness of injury, is less reliable in younger children and therefore makes comparison between children and adults challenging.
The most common cause of SCI in children and adolescents is motor vehicle crashes, followed by violence and sports. Unique causes of SCI in children include trauma resulting from lap belt injuries, child abuse, and birth injuries; and nontraumatic causes, such as instability of the upper cervical spine seen in Down syndrome, spinal stenosis seen in skeletal dysplasias, and inflammatory conditions, such as juvenile rheumatoid arthritis. The unique causes and pathophysiology seen in pediatric SCI are not only a consequence of small stature but are related to unique characteristics of the developing musculoskeletal system, which is exemplified by the phenomenon of SCI without radiographic abnormality (SCIWORA). In SCIWORA SCI, plain radiographs, computed tomography myelography, and dynamic flexion/extension studies are all normal, whereas MRI abnormalities are seen in approximately two-thirds of patients. Inherent elasticity of the spine in children allows transient self-reducing intersegmental displacement of the vertebral column in response to external forces and is believed to be the pathophysiologic mechanism underlying SCIWORA. Hyperelasticity of ligaments and joint capsules also contributes to risk of SCI in neonates during difficult labor and delivery, as shown by a cadaveric study by Leventhal. The neonatal vertebral column was tolerant of stretch up to 5 cm (2 in) without disruption, but the spinal cord could withstand only 0.64 cm (0.25 in) of distraction without tearing. The incidence of SCIWORA decreases with advancing age and reduced elasticity of the vertebral column, with 64% of children age 5 years or younger at injury having SCIWORA compared with 19% to 32% of older children up to age 21 years.
Review by system
Neurologic
Spasticity
Spasticity is a component of the upper motor neuron (UMN) syndrome characterized by velocity-dependent resistance to passive range of motion resulting from an increase in tonic stretch reflexes and accompanied by exaggerated tendon jerks. Spasticity is not immediately apparent after complete SCI during the period of spinal shock. Spinal shock is a temporary loss of spinal reflex activity occurring below the zone of injury and is associated with flaccid paralysis and absent muscle stretch reflexes. The duration of spinal shock is variable but usually resolves within weeks of injury. Spasticity generally follows spinal shock but may be delayed for up to 2 months or more in some circumstances. SCI characterized by concomitant damage to the anterior horn cell, nerve root, or distal LMN (including cauda equina) are characterized by flaccid paralysis and may mitigate the development of spasticity.
Clinical assessment begins with a comprehensive clinical evaluation by an experienced interprofessional team: the foundation for treatment planning and decision making. Spasticity is not always undesirable, and the rehabilitation approach to its treatment must consider both the positive and negative effects. The art of spasticity management is determining what degree of spasticity may be tolerable and of functional benefit versus counterproductive and potentially injurious.
The management of spasticity must be individually tailored to achieve specific goals set forth and agreed on by both the patient and clinician team. Taking an incremental approach with a treatment hierarchy has traditionally been recommended. For example, after excluding potential nociceptive factors exacerbating spasticity, physical therapy and modalities are introduced before consideration of oral medications or injections. If less invasive options are insufficient, more invasive orthopedic and neurosurgical treatments, such as tendon lengthening surgery or intrathecal baclofen pump implantation, would be considered.
Therapy plays a critical role in management of spasticity. Interventions used often include range of motion, prolonged passive muscle stretching via splinting, and education in a home program. Evidence for long-term efficacy of passive physical treatments such as stretching is lacking, although regular standing on a tilt table or standing frame is frequently encouraged to stretch hip, knee, and ankle flexors. Passive stretching may be more effective when combined with other modalities, such as application of heat, cooling, neuromuscular electrical stimulation, or selective nerve blocks. Functional electrical stimulation (FES) using a cycle has also been shown to reduce spasticity and preserve range of motion. In addition, hydrotherapy has been shown to help reduce spasticity and the amount of antispasmodic medication needed.
Oral medications used for systemic treatment of generalized spasticity commonly include baclofen, diazepam, dantrolene sodium, tizanidine, and clonidine ( Table 1 ). These medications differ in their mechanism of action, and little is known about the pharmacokinetics/dynamics in children. The choice of which medication to use is often based on clinical experience, commencing at a low dose, with careful titration to optimal dose, and monitoring for adverse effects or detrimental changes in function, such as increased muscle weakness. Medications may be used in combination to achieve complementary or synergistic effects. Baclofen is usually the first choice, with diazepam often added as an adjunct to baclofen, initially at night to assist with sleep and reduce nighttime spasms. Clonidine or tizanidine may also be useful adjuncts to baclofen and are also initiated as a single nighttime dose, because of sedative side effects. Dantrolene may be preferred in spasticity related to supraspinal injury, such as traumatic brain injury, to avoid sedation caused by baclofen and diazepam, although concerns about liver toxicity limit its use and necessitate monitoring of hepatic function.
| Oral Medication (Dose/Frequency, Age/Weight Range) | Mode of Action | Side Effects/Precautions |
|---|---|---|
| Centrally acting, structural analogue of GABA, binds to GABA B receptors of presynaptic excitatory interneurons (and postsynaptic primary afferents), causing presynaptic inhibition of monosynaptic/polysynaptic spinal reflexes Rapid absorption, blood level peaks in 1 h, half-life 3–4 h Renal and hepatic (15%) excretion | CNS depression (sedation, drowsiness, fatigue), nausea, headache, dizziness, confusion, euphoria, hallucinations, hypotonia, ataxia, paresthesia Note: abrupt withdrawal may cause seizures, hallucinations, rebound muscle spasms and hyperpyrexia |
| Centrally acting, binds to GABA A receptors mediating presynaptic inhibition in brain stem reticular formation and spinal polysynaptic pathways Rapid absorption, blood level peaks in 1 h, with half-life of 20–80 h Metabolized in liver, producing pharmacologically active metabolites with long duration of action. Increased potential for side effects with low albumin levels because of being 98% protein bound | CNS depression (sedation, impaired memory and attention), ataxia Dependence/potential for substance abuse/overdose Withdrawal syndrome (including anxiety, agitation, irritability, tremor, muscle twitching, nausea, insomnia, seizures, hyperpyrexia) |
| Peripheral action, blocking release of calcium from sarcoplasmic reticulum with uncoupling of nerve excitation and skeletal muscle contraction Blood level peaks in 3–6 h (active metabolite 4–8 h), with half-life of approximately 15 h Metabolized largely in liver, with 15%–25% of nonmetabolized drug excreted in urine | Malaise, fatigue, nausea, vomiting, diarrhea, muscle weakness with high dose Note: hepatotoxicity (baseline liver function tests must be checked before starting dantrolene, tested weekly during dose titration and regularly every 1–2 mo thereafter). Drug should be discontinued promptly if liver enzyme levels become increased |
| Centrally acting, α 2 -adrenoceptor agonist activity at both spinal and supraspinal sites. Prevents release of excitatory amino acids, facilitating presynaptic inhibition Good oral absorption, blood level peaks in 1–2 h, with a half-life of 2.5 h Extensive first-pass hepatic metabolism with urinary excretion of inactive metabolites | Dry mouth, drowsiness, tiredness, headache, dizziness, insomnia, anxiety, aggression, mood swings, visual hallucinations, risk of hypotension (although 10 times less antihypertensive potency than clonidine), nausea, vomiting and constipation Liver function tests should be monitored for increase in enzyme levels in view of possible hepatotoxicity |
| Centrally acting, mixed α-adrenoceptor agonist with predominant α 2 activity causing membrane hyperpolarization at multiple sites in brain, brainstem, and dorsal horns of spinal cord. Inhibition of substance P may also contribute to tone reduction via an antinociceptive effect Rapidly absorbed orally, blood level peaks in 1–1.5 h, with a half-life of 2.5 h | Drowsiness, dry mouth, bradycardia, orthostatic hypotension. Abrupt cessation may result in rebound hypertension |
Focal hypertonicity can be managed with intramuscular botulinum toxin (BTX) injections and phenol/alcohol neurolysis. Injections are most effective in children with dynamic muscle shortening caused by spasticity or dystonia that is localized to specific muscles and in children without significant contracture. Children undergoing injection with BTX may require sedation for comfort if multiple muscles are injected. In contrast, neurolysis with phenol is a more technically difficult procedure, requiring precision to avoid sclerosis of surrounding structures, and is more likely to require sedation. Electrical stimulation or ultrasonographic guidance may be used for muscle localization when injecting BTX in muscles that are not easily palpated, whereas phenol requires a targeting method for injections to avoid iatrogenic injury.
BTX blocks signal transmission at the neuromuscular junction by preventing the release of acetylcholine from the presynaptic axon of the motor end plate. BTX has been shown to be effective in adults with SCI; however, there are no randomized studies or studies in children with SCI. BTX use in small muscles may be an important adjunctive therapy to increase the therapeutic effect of antispasticity medications and intrathecal baclofen, resulting in a more focal effect, thereby improving the use of orthoses and the effectiveness of rehabilitation programs in patients with SCI.
Dosing and dilution guidelines in children have not been established, but consensus statements, systematic reviews, and randomized controlled trials provide recommendations. Considerations used in dosing include the child’s weight, muscle size, location of muscle, and degree of spasticity. The period of clinically useful relaxation is typically 12 to 16 weeks, and it is recommended that injections be spaced at a minimum of 3-month intervals because of concern for neutralizing antibody formation. Adverse events related to BTX are rare and include injection-site pain and focal muscle weakness. Since 2009, the US Food and Drug Administration has required black box labeling on BTX products, cautioning that the effects of the BTX may spread from the area of injection to other areas of the body, causing symptoms similar to those of botulism. Coadministration of BTX and aminoglycosides or other agents interfering with neuromuscular transmission is contraindicated. Further research on use of BTX in children with SCI is needed to determine efficacy, dosing, and functional outcomes in this population.
Phenol perineural injections (or motor point blocks) have been used for several decades to reduce focal spasticity in patients with SCI. The low cost of phenol is a significant advantage over BTX, but the need for electrical stimulation guidance and general anesthesia in children may offset any cost savings ( Fig. 1 ). Duration of clinical effect varies between 3 and 18 months. Phenol neurolysis is typically used for large proximal muscles, such as the biceps brachii, hip adductors, and hamstrings, but can also be performed in the posterior tibialis and gastrocnemius muscles, with minimal risk of sensory dysesthesia. Phenol injections have been combined safely with BTXs in children, and this combination allows an increased number of injections at the maximal recommended dose during 1 procedure. Phenol has been shown to be safe in children, but transient sensory dysesthesias occur rarely and were reported at 0.4% in 1 study.
Surgical management should be considered when spasticity causes significant functional impairments that are refractory to more conservative management. Most research on pediatric surgical spasticity management involves children with cerebral palsy. These procedures are also effective and widely used in spasticity of spinal origin.
Intrathecal baclofen (ITB) therapy is highly effective in treating generalized spasticity in both children and adults. ITB is delivered to the intrathecal space via a catheter connected to an implanted pump in the abdomen. This method of delivery infers an advantage over oral baclofen, in that central nervous system depressive effects are minimized and dosages can be titrated to functional effect. A thorough patient assessment and careful selection are crucial for a successful outcome. Goals of treatment (whether they are to improve function, comfort, or care) need to be firmly established. Patient and family education and close follow-up are essential. Cost and maintenance can be prohibitive for some families. The battery life of the newer pumps is approximately 7 years. A screening lumbar intrathecal bolus dose of baclofen is recommended to evaluate responsiveness and impact on abilities, as well as adverse effects, but these trials are not performed universally. Catheter tips are typically positioned at C5-T2 in patients with spastic tetraplegia and T10-T12 in those with paraplegia. Both the 20-mL and 40-mL pumps measure 8.75 cm in diameter, but the 20-mL pump is 19.5 mm thick, whereas the 40-mL version is 26 mm thick. Depending on the reservoir size and the patient’s dosing regimen, the refill interval is usually between 2 and 6 months. The surgical technique of placing pumps subfascially (see Fig. 1 ) as opposed to subcutaneously ( Fig. 2 ) in children may result in better aesthetics and lower incidence of wound erosion.
Potential complications of ITB include infections, respiratory depression, cerebrospinal fluid (CSF) leaks, and catheter problems, such as disconnection, migration, or kinking. Pump malfunction is less common. In 1 study, the most common complications of surgery were catheter-related problems (31%), seromas (24%), and CSFs (15%). ITB has not been found to significantly affect the rate of progression of scoliosis in children with cerebral palsy, but no such studies exist in children with SCI. ITB withdrawal is a medical emergency and needs to be identified early and managed aggressively. Caregivers should have a home supply of oral baclofen and be instructed to administer it at first signs of baclofen withdrawal. Causes can include catheter problems, human programming error, failure to refill pump, or medication error.
Dysautonomia (autonomic dysreflexia and temperature regulation)
Lesions of the spinal cord at the T6 level and rostral result in autonomic nervous system dysfunction, manifesting as autonomic dysreflexia (AD) and impaired temperature regulation. The pathophysiology, symptoms, and criteria for diagnosis (systolic blood pressure increase of 20–40 mm Hg) of AD are the same in children and adults. More than half of children with T6 or higher injuries likely experience AD, with older children having typical symptoms, such as facial flushing, headaches, and piloerection; children younger than 5 years rarely show these signs and symptoms. The key distinctions are that children have different normative blood pressures, and they may have more difficulty communicating the sometimes vague or abstract symptoms associated with AD. Diligence in looking for signs of AD, timely intervention, and education in AD prevention for parents/caregivers and patients are tenets to avoiding the serious complications.
Impaired temperature regulation results from disruption of afferent input to thermoregulatory centers and impaired control of the distal sympathetic nervous system. As a result, persons with SCI are susceptible to a phenomenon known as poikilothermia, whereby the patient assumes the temperature of the outside environment, resulting in either hypothermia or hyperthermia. Young children may be particularly susceptible to this complication, because of their inability to educate other caregivers, who may be less familiar with the risk of environmental exposure in SCI.
Respiratory
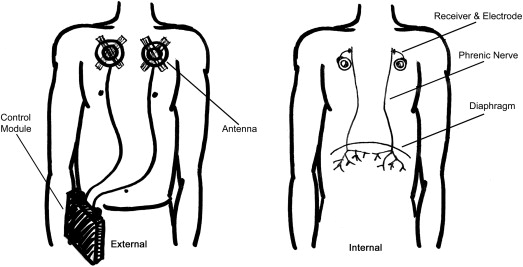
Respiratory complications from SCI are significant causes of morbidity and mortality in children. Although a child with cervical level SCI may require initial ventilator support, many of these children are weaned from this support. Children who are unable to be weaned require long-term ventilator support. This support can be provided by mechanical ventilator or, in some cases, by a phrenic pacer (see Fig. 2 ), which stimulates either the phrenic nerve or the diaphragm muscle, directly resulting in muscular contraction and resultant inhalation. Exhalation with the phrenic pacer is dependent on passive recoil of the chest wall, and, thus, with increased need for secretion management (such as in the setting of pneumonia) positive pressure ventilation is usually required. The advantages to phrenic pacing are that it is generally less invasive and mimics normal respiratory physiology more closely ( Fig. 3 ). Some children use a combination of both ventilation strategies.
Genitourinary
Neurogenic bladder
Similar to adults with SCI, the primary goals of neurogenic bladder management in children are to preserve renal function, prevent life-threatening complications, and promote continence. Clinical Practice Guidelines from the Consortium of Spinal Cord Medicine recommend use of clean intermittent urinary self-catheterization as the standard of care. Intermittent catheterization should be introduced at the age of 3 years, with the goal of obtaining complete independence by 5 to 7 years old, which mirrors the progression toward independent bladder management of the child’s able-bodied peers. Children who do not have sufficient hand function to catheterize independently should gain independence in verbal direction of care. The importance of obtaining social continence in SCI is paramount, because of the social stigma associated with foul odor and dependence on diapers, which may have devastating consequences for a child’s social development, self-esteem, and societal integration.
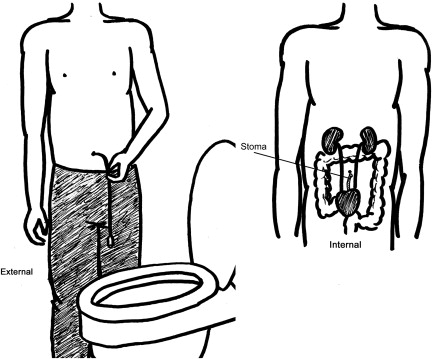
The success of neurogenic bladder management in achieving continence is dependent on the ability of the bladder to store sufficient urine until self-catheterization can be performed. For children who do not obtain satisfactory continence with conservative methods, surgical intervention may be a consideration. With a spastic detrusor muscle, anticholinergic medications, detrusor BTX injections, or bladder augmentation may be required to increase bladder storage capacity. Additional causes of incontinence may include an open bladder neck or weak urinary sphincter, resulting in stress incontinence. Incompetence of the bladder outlet may require urethral bulking injection agents, artificial urinary sphincter devices, or a bladder neck sling. In addition, in children with poor hand function, abductor strength, or females with difficulty mobilizing, a continent catheterizable urinary diversion ( Fig. 4 ) has shown improved independence with catheterization, greater overall patient satisfaction, and few complications.