Regardless of the cause or the location of the joint, the pathologic changes of arthritis have the greatest effect on the articular cartilage and the underlying bone. Initially, the soft tissue involvement includes only the synovium, but later it may involve, both directly and indirectly, the joint capsule, ligaments, and the adjacent tendons. The progression of arthritis is not only influenced by the existing disease processes, but also by the extent and rate of joint loading by mechanical forces.
The predominant clinical feature of arthritis is pain. For most of the major joints, the pain is based on the intraarticular changes of arthritis. Unlike other joints, however, the shoulder is more often affected by periarticular soft tissue disorders, which may coexist with glenohumeral arthritis and influence its clinical course. The functionally important musculotendinous cuff, along with the capsule and supporting ligaments, may undergo secondary changes as a result of glenohumeral joint arthritis. These include muscular weakness, pathologic musculotendinous shortening, and attenuation of ligamentous tissue. With the articular disease progression and the associated direct or indirect involvement of the unique soft tissue envelope of the glenohumeral joint, the clinical effect will be profound.
The glenohumeral arthritides and the associated disorders can be among the most challenging diagnoses. The clinician is required to use the acquired skills of careful history-taking, thorough physical examination, and prudent use of costeffective testing. The glenohumeral arthritis and its allied conditions include osteoarthritis, chondrolysis, posttraumatic arthritis, arthritis of dislocation, osteonecrosis, rheumatoid arthritis (RA), crystalline arthritis, rotator cuff tear arthropathy, noninfectious inflammatory arthritis, and miscellaneous arthropathies.
The shoulder pain is not only a very common symptom that clinicians are asked to evaluate, but also the chief complaint of patients with glenohumeral arthritis. Acute traumatic shoulder pain is reviewed elsewhere in this textbook. This chapter will highlight the major causes of glenohumeral arthritis and profile the characteristics of each disorder.
Clinical Presentation
Although the shoulder is no less susceptible than other diarthrodial joints to arthritic conditions, arthritis is not a typical cause of shoulder pain. Probably as few as 5% to 10% of patients with shoulder pain have arthritis as a part of a polyarticular systemic disease, or a monarticular disease process. Painful dysfunction is more often a disorder of one or more of the soft tissue elements of the shoulder.
There exist more than 100 different types of arthritis. They may be defined as inflammatory or degenerative, polyarticular or monarticular, and acute or chronic. Distinction is made from historical factors, physical findings, radiographic analysis, and tissue sampling, including the synovial fluid analysis and serum studies.
The predominant symptom of patients with arthritis is pain, usually intensifying with use and interfering with sleep. The shoulder motion is restricted by synovial membrane inflammation and increased fluid production. The synovium and capsule are richly innervated structures easily irritated by distention from the accumulation of synovial fluid. Exacerbation of discomfort with rotary movement of the elbow at the side is a characteristic physical finding of an inflammatory condition of the glenohumeral joint. Degenerative conditions are more tolerant of passive range of motion. Tenderness over the posterior joint line, as noted by Neer, is notable in glenohumeral osteoarthritis.
577 Tenderness over the anterior joint line, lateral and inferior to the coracoid process, is very suggestive of an inflammatory disease. It is not commonly elicited in rotator cuff disease or in noninflammatory conditions.
Imaging
Green and Norris have provided an excellent review of imaging strategies to be employed for patients with glenohumeral arthritis.
286 Initial evaluation of the painful shoulder will almost always include plain radiographs.
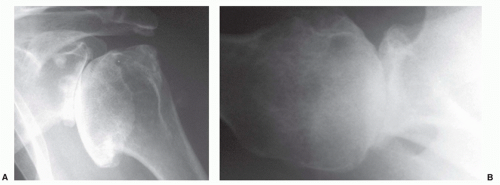
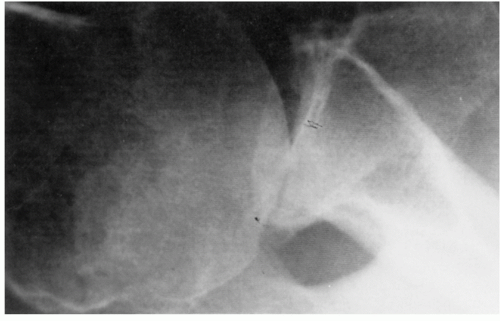
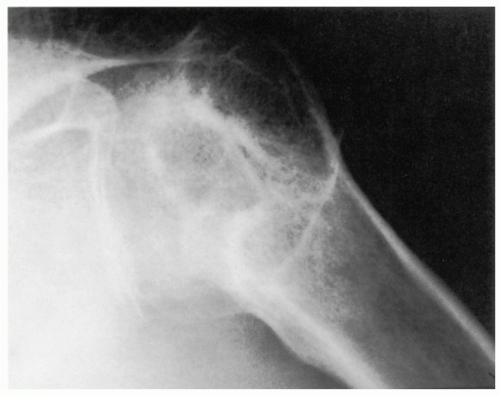
409 The views most commonly employed by the orthopedic surgeon are the true anteroposterior (AP) of the glenohumeral joint orthogonal to the plane of the scapula and the transaxillary lateral. Glenohumeral arthritis is easily distinguished from other soft tissue conditions and is classified based on features identified on plain films (
Fig. 12-1).
687 These include alignment and
relation of the humerus to the glenoid and acromion process, the width of the articular cartilage, osseous erosions, productive changes including osteophytes, and the presence of soft tissue swelling and calcification.
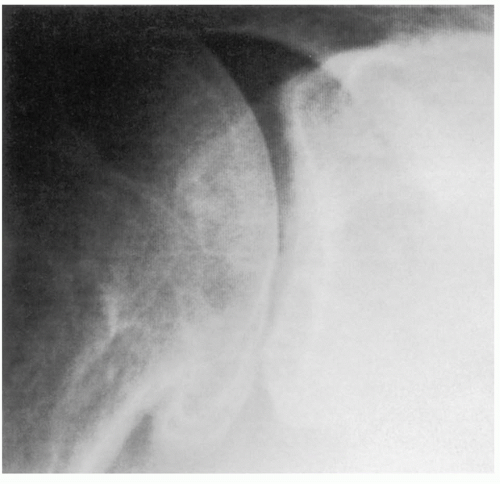
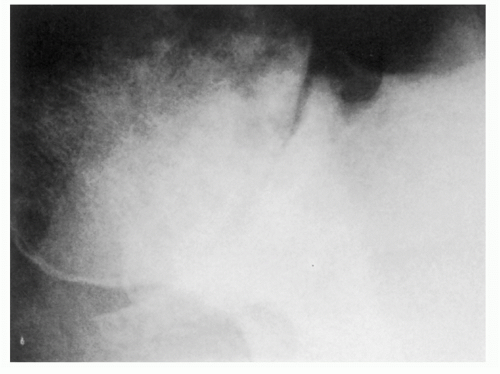
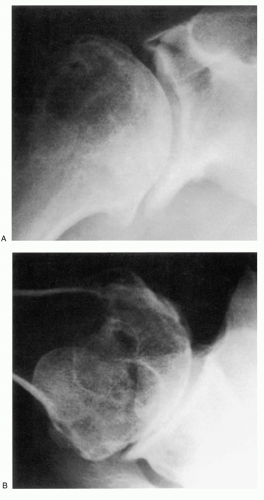
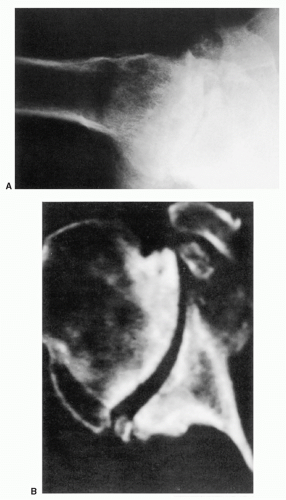
Distinction of the articular cartilage space is best made when the X-ray beam is directed tangent to the joint surface. For the shoulder, this requires accurate positioning of the patient, shoulder, and extremity. Once the plane has been determined, the humerus can be externally rotated to approximately 35 degrees (
Fig. 12-2). This will profile the load-bearing portion of the articular surface so that the earliest narrowing does not escape detection, and it is also the position favoring visualization of marginal humeral head osteophytes.
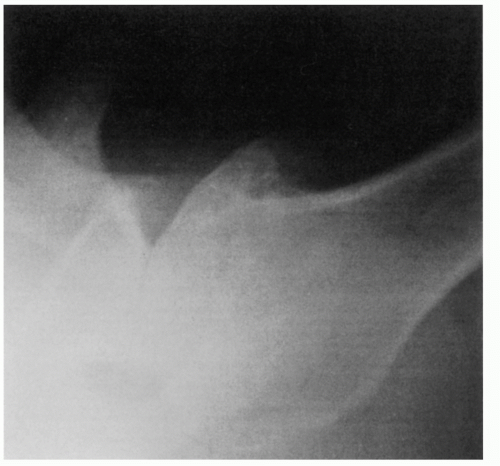
410 Apple et al. suggested the weighted abduction Grashey shoulder method as a more sensitive means of detection of loss of articular cartilage.
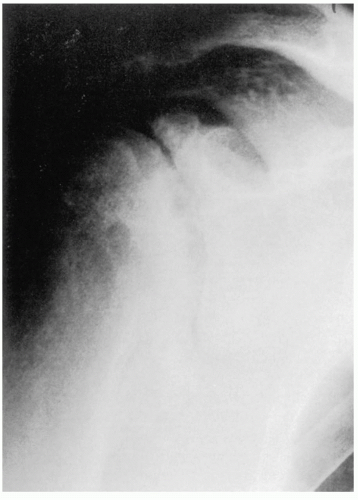
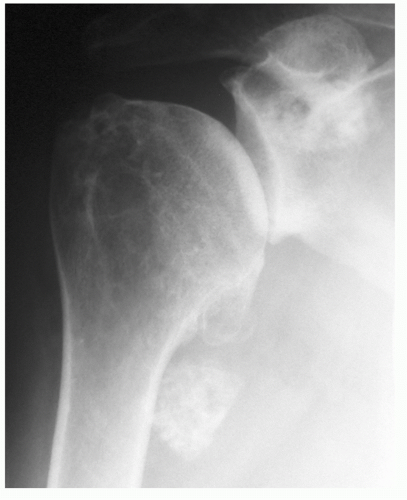
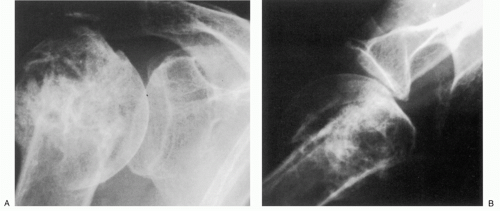
25 When the disease is in more advanced stage, positioning the humerus by external rotation is difficult and cannot be done exclusive of the position of the scapula (
Fig. 12-3). For Nelson et al., plain radiography, as opposed to magnetic resonance imaging (MRI), computed tomography (CT) arthrogram, and ultrasonography, was most useful for the diagnosis of osteoarthritis.
583 Atlases and classification systems have been proposed for the study and treatment of osteoarthritis.
14,
15 The Samilson-Prieto classification for glenohumeral arthritis offers advantages due to its simplicity and reliability.
84,
406,
715Although shoulder arthrography is employed mostly for the detection of rotator cuff tears, the articular surfaces and contours of the synovial lining of the joint can be easily visualized.
802 The arthrographic features may be enhanced by the use of poly-tomography or CT.
456,
742 Filling defects may be observed in the cartilaginous surfaces, and the thickness of the residual cartilage is estimated. Magnetic resonance arthrography using gadopentetate dimeglumine may offer a more sensitive method for the detection of intra-articular abnormalities.
432,
610 These techniques, for practical purposes, are rarely employed by the author for the routine assessment of glenohumeral arthritis.
The ultrasound study of the glenohumeral articulation has a very limited, yet undefined, role in the evaluation of the articular surfaces. It may prove more useful in the detection of synovitis and periarticular soft tissue disorders.
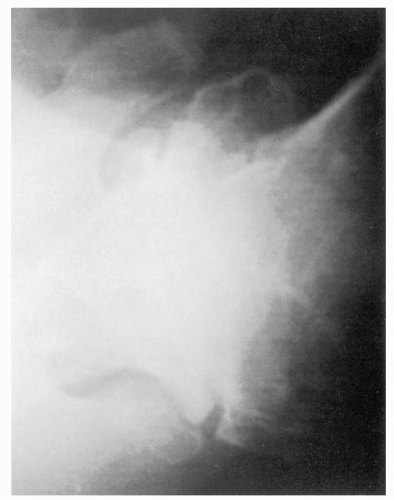
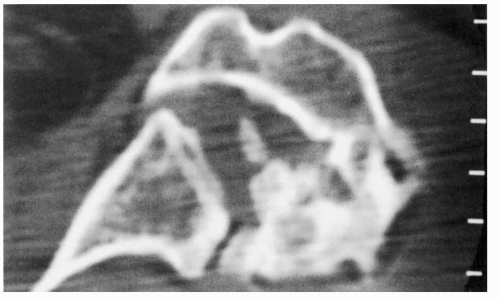
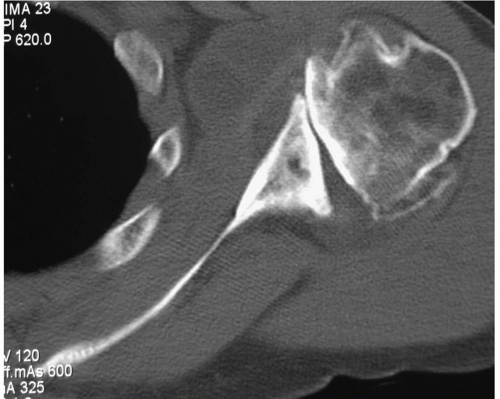
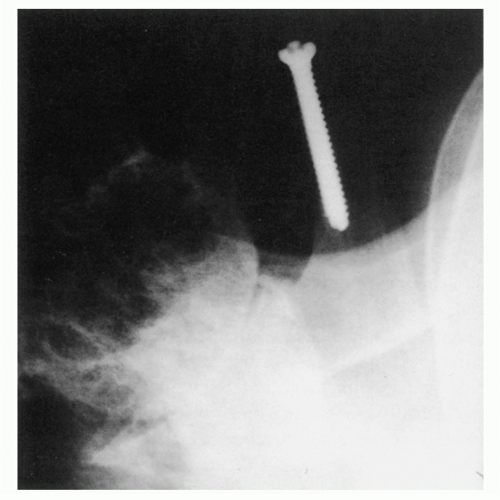
366Although a CT scan is generally not required for the diagnosis of articular disorders, it can be quite helpful to delineate the extent of bone involvement and to assess glenoid orientation (
Fig. 12-4).
251,
561 Glenohumeral morphology can be detailed, as can the quality and quantity of the glenoid. CT scanning will also help detect subtle cortical erosions, deposits of calcium, and the presence of newly formed bone. A CT scan may more accurately assess glenohumeral relationships when extreme stiffness or deformity does not allow the shoulder to be positioned for optimal plain film study. Combining fluorine-18-fluorodeoxyglucose (FDG) positron emission tomography (PET) with CT proved 100% reliable for the detection of osteoarthritis and RA in patients with shoulder pain.
552 FDG accumulates diffusely about the shoulder in patients with clinical symptoms and signs of osteoarthritis, although false-positive scans are not infrequent.
844Perhaps, the best imaging study for detecting early chondral injury or damage is MRI, even though the assessment of the articular cartilage can be technically challenging.
274 Monographs to which the readers are referred to give the details on MRI of articular cartilage and osteoarthritis.
370,
684 Hayes stated that an accurate MRI scan of articular cartilage requires
good spatial resolution for the detection of small defects, good subject contrast and image contrast for the detection of signal intensity changes in articular cartilage, reliable distinctions for the detection of signal intensity changes in articular cartilage, and reliable distinction between articular cartilage and adjacent subchondral bone and joint fluid.
323 Broderick’s study drew attention to the tendency for cartilage abnormalities to be underestimated with MRI when compared with arthroscopic observations.
77 Amin made a similar observation when MRI was compared with radiographic progression.
17 The use of injectable contrast agents to enhance the detection accuracy of articular cartilage is not generally necessary.
118,
433,
523There is a consensus that normal articular cartilage can be imaged by MRI, perhaps to the extent of “zonal” analysis.
143,
546,
617,
703 There are several means of assessing articular cartilage morphology using MRI techniques with each having advantages and disadvantages in the quest to distinguish articular cartilage from subchondral bone.
195,
266,
469,
642,
679 Three-dimensional imaging data can provide information about joint surface topography utilizing refined techniques.
33,
117,
622,
623,
667,
797 Quantitative MRI is under investigation as a means of earlier detection and as a tool for monitoring the response of the articular cartilage to certain clinical conditions and to treatment.
22,
85,
150,
205,
206,
207 and
208,
257,
302,
654,
829 Advances have been made in cartilage matrix assessment using MRI quantitative dGEMRIC, diffusion tensor imaging, sodium MRI, T2 and T1rho mapping techniques.
98,
236,
242,
271,
469,
534,
669,
776,
851,
859 Presently, the advantages of dGMERIC are recognized and its use is more widespread.
61,
563,
801,
851 T2 mapping with dedicated software is more sensitive than routine MRI for the detection of early pathological changes in articular cartilage.
494With a few exceptions, the earliest scientific studies of MRI of articular cartilage involved the knee.
65,
143,
296,
301,
345,
523,
524,
622,
667 The trend continues currently.
195,
326,
584,
671,
685,
686 Huber, in one of the earliest published reports on MRI of the normal shoulder, cited the failure of MRI to effectively define articular cartilage.
355 The literature was replete with studies of MRI of the shoulder that had assessed features of the rotator cuff and capsulolabral morphology in impingement and instability disorders, respectively, and rarely mentioned the findings of the glenohumeral articular cartilage. Recent monographs and textbooks concerned with MRI of the shoulder and shoulder imaging inadequately address arthritis imaging by MRI, particularly the articular cartilage changes.
753,
814,
891 The preferred timing, sequences, and technical parameters have not been determined with uniform success and are evolving.
344 Therefore, MRI applications for glenohumeral articular disorders are not yet as extensive as for the knee. Zlatkin stated that MRI is rarely required to assess patients for glenohumeral osteoarthritis alone.
892 MRI arthrography utilizing gadolinium compounds may prove to be an effective technique to enhance the glenohumeral articular surfaces.
295,
433A shortcoming of conventional radiographs is failure to detect soft tissue details, although in some cases swelling can be identified. Joint effusions may be suspected on plain films, but are best evaluated by ultrasound, CT, or MRI. The MRI is probably the most useful, accurate, reliable, and expensive method.
814,
864In normal shoulders, the MRI findings show only a thin rim of joint fluid around the biceps tendon sheath and narrow bands in the axillary recess and subscapularis bursa; the volume has been observed to be increased with age.
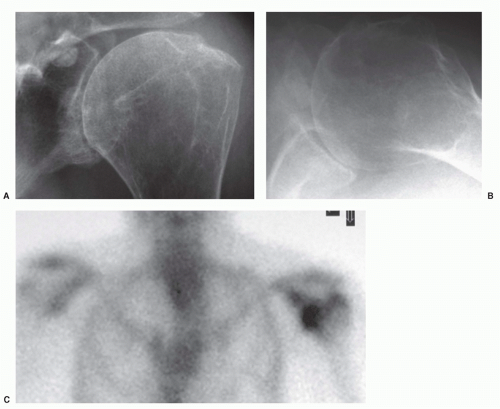
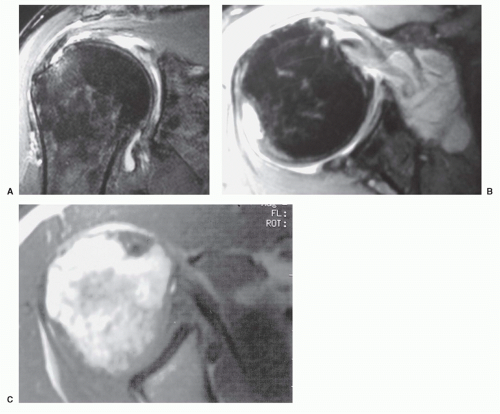
743 The accumulation of glenohumeral joint fluid is abnormal; usually it is related to rotator cuff tears or osteoarthritis (
Fig. 12-5A,B).
743 The intravenous administration of gadopentetate dimeglumine will enhance the MRI of joint fluid, although the response is slower and less vigorous than synovium.
863The use of MRI allows direct visualization of inflammatory synovitis and synovial proliferation within the joint, as well as its penetration into the adjacent bone and periarticular soft tissues (
Fig. 12-5C).
673,
865 Contrast enhancement with the use of gadolinium provides a more specific assessment of synovitis or pannus.
430,
673 The rate and intensity of synovial enhancement may vary, depending on the activity of the synovium as a reflection of the activity of the disease process, as well as the image timing.
863Radionuclide isotope uptake in tissue reflects the rate and extent of blood flow through the tissue and, in bone, is a measure of bone metabolism, especially osteoblastic activity. Scintigraphy, in particular triple-phase bone scan, may prove useful in distinguishing soft tissue inflammation from osteoarticular changes.
270,
495,
811 It is very sensitive, but it does not distinguish acute inflammatory disease from bone and joint sepsis.
810 Technetium scans have been used to confirm the presence of RA before the appearance of radiographic abnormalities. 189 Indium-111 chloride has been used for the detection of RA as well as for following the course of disease activity.
732,
752 Immune complex scintigraphy holds promise for monitoring disease activity and response to treatment.
171,
415 Uptake defects can exist in osteonecrosis and can be enhanced with single photon emission computed tomography images.
812,
849 White cell-labeled scans are useful when an infectious disease process is being considered.
811,
833
Laboratory
Serum studies may be helpful, but only rarely can a diagnosis be obtained from this information alone. Complete blood count, autoantibodies (rheumatoid factor [RF] and antinuclear antibody), uric acid and acute-phase reactant measurements, erythrocyte sedimentation rates (ESRs), and C-reactive protein (CRP) are most often performed as an initial screening battery and are most helpful when the diagnosis is in question.
258,
736 Acute-phase reactants are serum proteins formed in the liver and include coagulation, transport, complement, and miscellaneous reactive proteins. Their production is accelerated in the presence of inflammatory states and tissue necrosis.
259,
441,
737 The ESR is a rough, indirect quantification of their serum levels. The CRP level has been used more recently. Both are serum markers that reflect the extent or degree of inflammation and can be used for the assessment of disease activity or response to treatment over time.
142,
441 The CRP levels (normally less than 1 mg/dL) may exhibit moderate elevation (1 to 10 mg/dL) in most of the connective tissue diseases; marked elevation (more than 10 mg/dL) may signify an acute bacterial infection such as septic arthritis.
553Clinically detectable effusions are uncommon in most arthritides. When present or suspected, however, a fluid sample should be obtained and submitted for analysis.
786 It should also be obtained any time the patient’s history, physical examination, and radiographs support the diagnosis of arthritis but a diagnosis has yet to be determined.
261 The findings of joint fluid analysis have been shown to change a clinically suspected diagnosis, and often treatment, in 20% of samplings.
215 Particularly in acute arthritis, synovial fluid analysis is of major diagnostic value.
786Arthrocentesis of the shoulder can be performed from the anterior or posterior approach. Anteriorly, the tip of the coracoid process is palpated. After local anesthetic instillation, an 18- or 21-gauge spinal needle is passed through the deltoid muscle at a point approximately 1.5 cm inferior and 1.5 cm lateral to the tip of the coracoid process. The cephalic vein is nearby and may be inadvertently punctured. Entry into the joint is gained with passage through the subscapularis muscle and the joint capsule. Posteriorly, the point of entry is two to three fingerbreadths inferior and one to two fingerbreadths medial to the posterolateral corner of the acromion process.
Directed toward the coracoid process, the needle will penetrate the deltoid and infraspinatus muscles to enter through the capsule into the joint.
As much fluid as possible is withdrawn and its volume is determined. It is characterized as shown in
Table 12-1.
146 The gross characteristics are noted. These include viscosity and color, which is normally clear and yellowish tinged. The fluid is typically not bloody, although blood tingeing may suggest a traumatic tap or the existence of a pathologic lesion.
Fluid in normal joints is present in quantities that may preclude sampling by arthrocentesis. Recht detected fluid in 14 of 20 shoulders by MRI in 12 asymptomatic young volunteers; in none was more than 2 mL evident.
668 If the joint fluid is obtained, the white count is less than 200 and predominantly monocytic as opposed to polymorphonuclear, and there are no red cells.
261Additional joint fluid types have been noted.
261 Noninflammatory fluid has a white cell count usually less than 2,000.
146 The fluid is transparent or nearly so. This type of fluid can be seen in trauma, osteoarthritis, systemic lupus erythematosus (SLE), sarcoid, and hypothyroidism.
Inflammatory fluid may show varying degrees of clarity and color. The white cell count is more than 2,000, but usually not more than 50,000.
146 This is commonly seen in RA, gout, and possibly some infectious disorders. It is also associated with Reiter’s syndrome, ankylosing spondylitis, psoriatic arthritis, and juvenile RA.
Pyogenic fluid is opaque or grossly purulent. The white cell count exceeds 50,000, often higher, and is predominantly polymorphonuclear cells.
146 This is typical of infectious arthritis and, in some cases, gout or other very inflammatory arthritides.
Nonpyogenic fluid has variable characteristics. The white blood cell count is usually less than 20,000.146 Additionally, joint fluid can be characterized as hemorrhagic. This will occur in cases of trauma, hemophilia, and other bleeding disorders.
Microscopic identification of crystals is a laboratory test with notable problems in sensitivity, specificity, and interobserver differences.
123,
693,
747 Despite this observation, the fluid should routinely be examined for calcium pyrophosphate (CPP) and uric acid crystals that are further characterized by polarized light microscopy.
614 Gout crystals are pointed and negatively birefringent.
630 CPP crystals are rhomboid and positively birefringent.
262,
395Synovial fluid glucose levels are normally approximately 20 mg/dL less than the serum. Lower concentrations may be
observed in joint sepsis. Synovial fluid protein is often increased in inflammatory disease. The utility of synovial fluid glucose and protein levels is questionable.
786 Appropriate organism stains and cultures should be obtained when there is even the most remote suspicion of an infectious process.