Pathology of Osteoarthritis
Aubrey J. Hough Jr.
Historical Concepts of Osteoarthritis
Medical science is to a degree inseparable from its ancient origins in so-called natural philosophy. Disease itself was originally regarded as the visitation of evil recompense, often for specific sins of omission or commission. Thus, the naming of diseases took on a semantic basis reflecting primitive ideas about causation as well as symptoms. In some ways, the naming of diseases was originally analogous to the naming of other perceived manifestations of evil, such as demons, before exorcism. In other words, the name of the disease must necessarily precede its cure. Hence, the Latin noun rheuma, denoting a fluid exudation, became the linguistic source for rheumatism. This illustrated both a principal external sign of joint disease, swelling, and the theoretical consideration of imbalance in fluid (phlegm) derived from the galenic humoral theory of disease.
It was not until the period of the enlightenment (1600–1760) that serious scientific attempts were made to subclassify accurately the various forms of arthritis known to us today. In the nineteenth century, subsequent accumulation of scientific and clinical evidence led to the first delineations of specific arthritic disorders,1 such as gout and rheumatoid arthritis (Alfred B. Garrod, circa 1858-1876) and ankylosing spondylitis (Adolph Strumpell, 1897; Pierre Marie, 1898). Morbid anatomy played an important part in these discoveries. Perhaps owing to the lack of systemic symptoms in most cases, the entity now known as osteoarthritis emerged fairly late as a distinct disorder, although specific manifestations, such as Heberden (1802) and Bouchard (1884) nodes, were described before recognition that they were associated with a specific disease state. Although several individuals had earlier observed localized erosion of cartilage, especially in relation to deforming lesions of the hip, Archibald E. Garrod (1907) was the first to clearly distinguish rheumatoid arthritis from osteoarthritis. The twentieth century emergence of more unitary concepts of osteoarthritis has been reviewed in detail by Sokoloff.2 Understanding of the significance of the pathologic events on a molecular basis has amplified, rather than reduced the apparent complexity of the disorder and increased the need for careful consideration of sequential pathologic changes in its evolution.
Definitions and Terminology
The definition and terminology of osteoarthritis have long been the subject of conjecture, debate, and, at times, some degree of polemics. Even before the impact of molecular biology on medicine, Tarnopolsky3 identified 54 different names for the entity, and the intervening half-century has produced more. The term osteoarthritis has gained primacy through long use in the English-speaking medical community, but it is less than satisfactory because of the implicit connotation that inflammation is the root cause of the disorder. Interestingly, osteoarthritis was the term originally proposed by John Spender in 1886 as a more suitable name for rheumatoid arthritis.1 The terms osteoarthrosis and degenerative joint disease have a certain appeal but are nonspecific. Furthermore, they give no information about the pathologic processes that characterize the disorder. Arthritis deformans, as proposed by Heine4 in 1926, was for many years considered a synonym for osteoarthritis in the European medical community. However, this usage primarily reflects the gross proliferative changes seen in advanced cases of primary osteoarthritis, particularly of the hip joint, and neglects the contributions of early destabilizing events that occur in either the articular cartilage or underlying subchondral bone. Articular cartilage changes did not come to the forefront until in the investigations of
Bennett, Waine, and Bauer5 in 1942. In a far-reaching career, based almost entirely on pathologic examination, Johnson6 promulgated the concept that osteoarthritis represents a decompensated remodeling of the joint in response to chronic biomechanical stress. That view persists, in at least modified form, to this day.
Bennett, Waine, and Bauer5 in 1942. In a far-reaching career, based almost entirely on pathologic examination, Johnson6 promulgated the concept that osteoarthritis represents a decompensated remodeling of the joint in response to chronic biomechanical stress. That view persists, in at least modified form, to this day.
One current definition of osteoarthritis7 takes into account “morphologic, biochemical, molecular and biomechanical changes of both cells and matrix which lead to softening, fibrillation, ulceration and loss of articular cartilage, sclerosis and eburnation of subchondral bone, osteophytes, and subchondral cysts.” This chapter lays out the pathologic basis of osteoarthritis and current concepts of the role of pathology in understanding its pathogenesis.
Microscopic Anatomy of Normal Joints
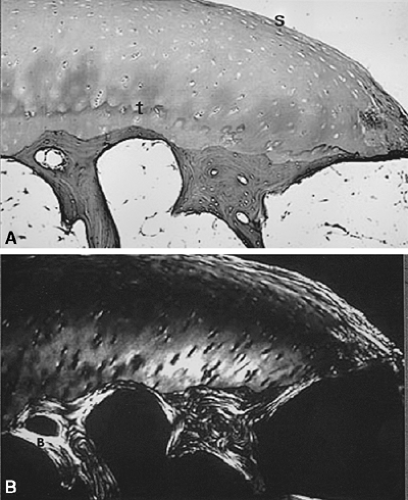
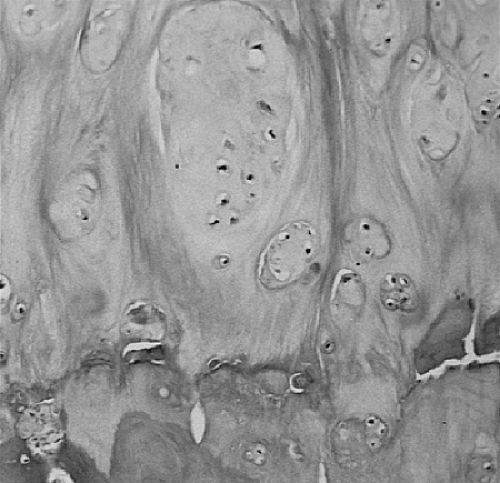
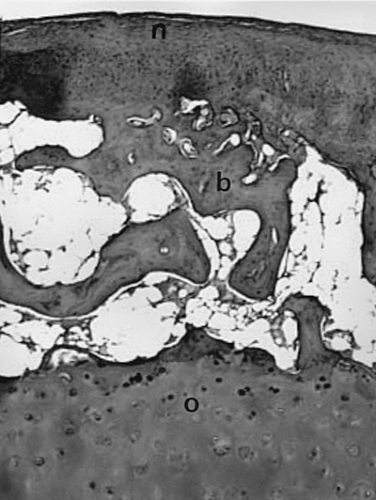
The typical diarthrodial synovial joint has two opposing surfaces composed of hyaline articular cartilage. The cartilage is composed of four distinct zones. These are a superficial tangential zone characterized by chondrocytes and collagen fibers aligned roughly parallel to the surface, an intermediate zone, a deep radial zone with chondrocytes and collagen fibers aligned perpendicular to the surface, and a zone of calcified cartilage firmly joined to the underlying subchondral bone (Fig. 3-1A). The arrangement of the collagen fibers can be clearly appreciated when a section is illuminated with plane-polarized light (Fig. 3-1B). The junction of the zone of calcified cartilage with the deep radial zone is marked by an undulating hematoxyphilic line that is visible even in decalcified sections. The junction of this zone with subchondral bone is abruptly demarcated by a cement line into which fibers insert. This is seen clearly in electron micrographs (Fig. 3-2). Under normal conditions, the zone of calcified cartilage is slowly replaced by bone surrounding vascular in growths penetrating from the underlying subchondral bone marrow, but remodeling of this zone is an early feature of osteoarthritis.8
The viscoelastic behavior of cartilage is dependent on the water-binding properties of the matrix protein-polysaccharide moieties, which are in turn contained within a meshwork of collagen as illustrated in Figure 3-1b. The meshwork is predominantly the unique monomeric type II collagen that is enriched with hydroxylysine, but other minor collagens, such as type VI and type IX, are also present. Type IX collagen has been postulated to act as a link between proteoglycans and type II collagen. Type X collagen, itself more characteristic of epiphyseal cartilage, is found in calcified zones containing hypertrophic chondrocytes. Specific immunocytochemical procedures can demonstrate these collagens both in the normal state and in pathologic changes accompanying osteoarthritis. The marginal tissues of normal diarthrodial joints contain some areas of fibrocartilage, easily detectable because of the larger diameter of the collagen fibers contained therein and the reduced content of metachromatic proteoglycans compared with hyaline cartilage. Proliferative cartilage associated with osteoarthritis is frequently overtly fibrocartilaginous in appearance, adding to the heterogeneity that exists even in the normal state.
Current Hypotheses Regarding Pathologic Lesions
Most current ideas devolve from the concept that osteoarthritis arises from a chain of events leading to abnormal remodeling. Remodeling in this sense results in gradual removal of “old bone” at some sites and simultaneous production of “new bone” at others. This goes on normally with aging, but that which occurs in osteoarthritis is both qualitatively and quantitatively different from the normal situation in that it is both aberrant and progressive.
The maintenance of homeostasis in cartilage is analogous to that in bone; experimental models demonstrate loss of cartilage matrix in areas of decreased pressure and necrosis of chondrocytes in areas of increased pressure.9 Similar loss of matrix with resultant thinning of articular cartilage is observed in humans in hip joints of patients with spastic cerebral palsy. Both chondrocyte10 and osteocyte11 death have been described in human osteoarthritis. Likewise, the view that fibrillation or denudation of cartilage always precedes bone remodeling is problematic. Fibrillation and remodeling of the basal calcified cartilage
often coexist but in different portions of the articular surface. Remodeling is prominent in non-weight-bearing areas with fibrillation occurring in the weight-bearing zones.12 Microfractures13 of the calcified cartilage (Fig. 3-3) themselves contribute to cartilage destruction by allowing vascularized marrow elements to penetrate the articular cartilage8 thereby promoting dissolution or ossification in the cartilage. Subchondral bone remodeling of microfracture14 may in itself promote cartilage destruction by increasing the stiffness of the underlying bone. In this view, repair of microfractures may cause the cartilage to absorb a greater portion of the energy impacting the joint.15 The situation is clearly complex. However, disordered remodeling is the source of much of the pathology seen in progressive osteoarthritis.
often coexist but in different portions of the articular surface. Remodeling is prominent in non-weight-bearing areas with fibrillation occurring in the weight-bearing zones.12 Microfractures13 of the calcified cartilage (Fig. 3-3) themselves contribute to cartilage destruction by allowing vascularized marrow elements to penetrate the articular cartilage8 thereby promoting dissolution or ossification in the cartilage. Subchondral bone remodeling of microfracture14 may in itself promote cartilage destruction by increasing the stiffness of the underlying bone. In this view, repair of microfractures may cause the cartilage to absorb a greater portion of the energy impacting the joint.15 The situation is clearly complex. However, disordered remodeling is the source of much of the pathology seen in progressive osteoarthritis.
Articular Cartilage in Osteoarthritis
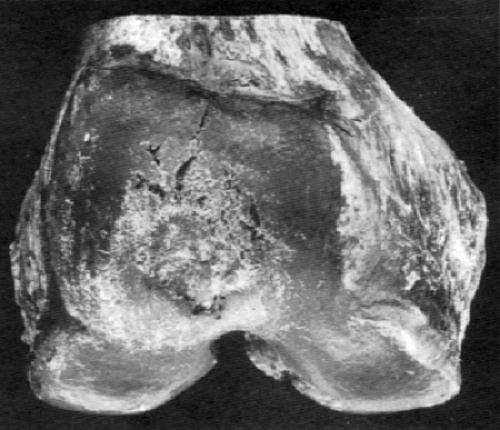
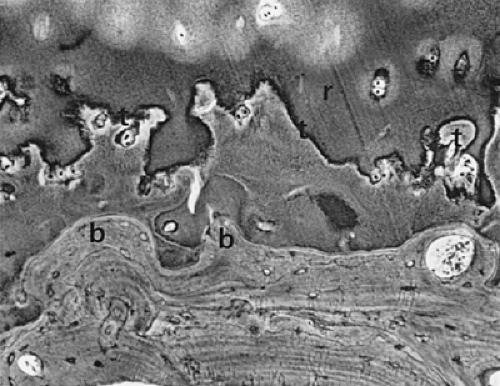
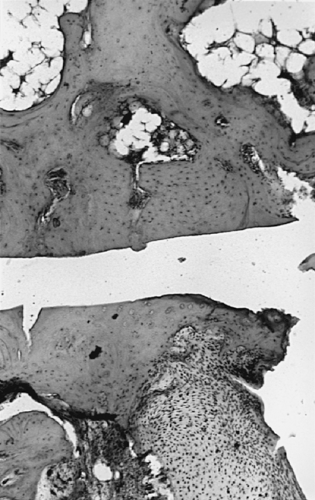
Any consideration of the pathologic process of osteoarthritis begins with articular cartilage. As a nonvascularized tissue, articular cartilage displays a limited number of response patterns to injury. Fibrillation, characterized by vertically oriented superficial dehiscence of the extracellular matrix, is apparent in most cases of early osteoarthritis examined at autopsy. This gives the cartilage the gross appearance of velvet rather than the normal glistening smooth appearance. Many of these examples of fibrillation apparently do not progress to clinically significant osteoarthritis. However, fibrillation is frequently seen in association with osteoarthritis, albeit in a pattern different from that described previously (Fig. 3-4). On microscopic examination, the fibrillation in osteoarthritis is usually associated with deeper clefts, more obvious dissolution of matrix, and chondrocyte proliferation in response to injury. A more pronounced stage of cartilage injury, less common than fibrillation, is known as cracking. Here, the vertical dehiscences are deeper, often extending into the zone of calcified cartilage, and are accompanied by a horizontal component as well (Fig. 3-5). Similar changes have been described in degenerating meniscal fibrocartilage.16 This type of cracking can be seen macroscopically and is associated with erosion of cartilage from the loaded or weight-bearing areas that is characteristic of progressive osteoarthritis (Fig. 3-6).
Chondromalacia or softening of articular cartilage has been associated with fibrillation and has been described as an early change in progressive osteoarthritis. It is particularly prominent in precocious patellofemoral osteoarthritis (chondromalacia patellae) of younger individuals. However, differences in the histologic findings exist between chondromalacia of young adults and progressive osteoarthritis.17 In addition, chondromalacia patellae without malalignment often does not progress to clinically
significant osteoarthritis18 and the disorder is related more strongly to injury than osteoarthritis.19 Chondromalacia is also associated with changes in the type and content of proteoglycans.20 This predisposes the cartilage to erosion with exposure of underlying bone in severe examples. The loss of matrix proteoglycans also exposes the collagen fibrils so that they may undergo disaggregation21 and release type II collagen fragments.22
significant osteoarthritis18 and the disorder is related more strongly to injury than osteoarthritis.19 Chondromalacia is also associated with changes in the type and content of proteoglycans.20 This predisposes the cartilage to erosion with exposure of underlying bone in severe examples. The loss of matrix proteoglycans also exposes the collagen fibrils so that they may undergo disaggregation21 and release type II collagen fragments.22
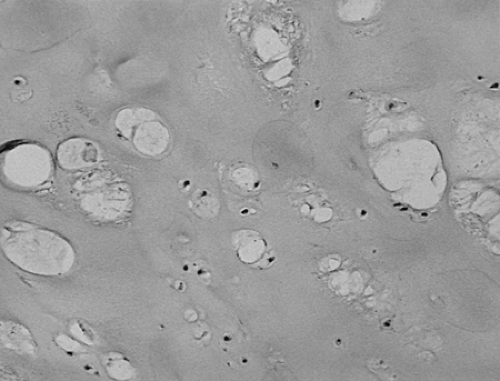
Early accounts5 noted the increased numbers of chondrocytes adjacent to areas of chondromalacia and the increased affinity of the perilacunar matrix for hematoxylin. More recent studies have confirmed an increase in the synthetic activities of chondrocytes, including collagens23 and degradative enzymes,24 in these zones. Although apoptosis of chondrocytes in osteoarthritis has been documented,25 there is an obvious increase in numbers of chondrocytes characterized by multiple cells per lacuna adjacent to zones of fibrillation and chondromalacia (Fig. 3-7). Notwithstanding that metaplastic chondrocytes in osteoarthritis might be capable of migration to produce this appearance,26 the preponderance of evidence supports focal mitotic activity as the basis of the “clones.”27 The proliferating chondrocytes associated with erosive lesions have been demonstrated to contain unstable DNA with tetraploidy.28 Other studies have identified trisomy 7 as a characteristic acquired somatic mutation in osteoarthritic synovia and cartilage.29 Enlarged, phenotypically altered chondrocytes are also seen, particularly in the deeper zones (Fig. 3-8). These cells have been shown to produce type X collagen, normally associated with the hypertrophic zone of epiphyseal cartilage.30
The proliferation of chondrocytes has long been associated with the perilacunar dissolution phenomenon known as Weichselbaum lacunar resorption. This change (Fig. 3-9) was once identified as relatively specific for early osteoarthritis but is also seen in rheumatoid arthritis. It is best regarded as a histologic expression of degrading of cartilage matrix by chondrocytes responding to cytokines, recently described quanitatively.31
Suppression of anabolic activities, particularly those of matrix protein synthesis, has been demonstrated in upper zones of osteoarthritic cartilage.32 Secretion of several matrix metalloproteinases is increased over that in normal cartilage,33 particularly in response to tumor necrosis factor-α (TNF-α)33 or interleukin-1a.33 Consequent release of type II collagen fragments correlates with the progression of osteoarthritis.34,35
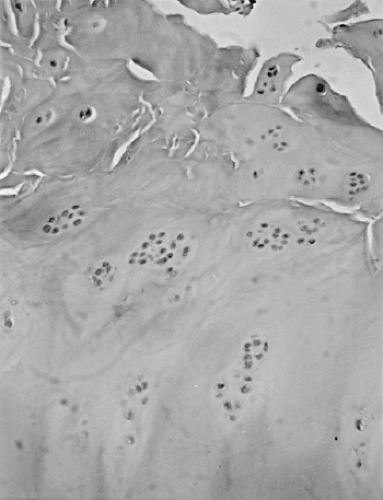 Figure 3-7 Photomicrograph from osteoarthritic proximal tibial articular surface demonstrates chondrocyte proliferation adjacent to area of fibrillation. (Magnification x 50.) |
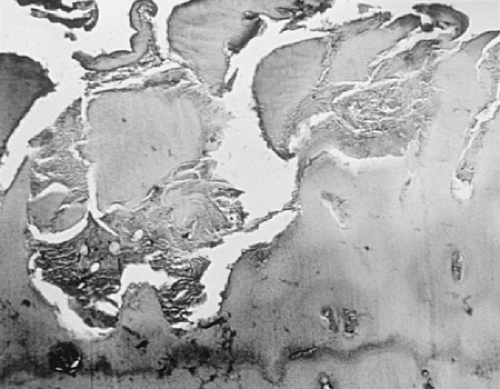
In osteoarthritis the original hyaline articular cartilage is partially replaced in progressive disease by a repair cartilage (Fig. 3-10). This cartilage often overlies a deeper zone of original articular cartilage (Fig. 3-11). Frequently, this repair cartilage has the histologic and histochemical characteristics of fibrocartilage, containing obvious broad collagen fibers and reduced amounts of matrix proteoglycans compared with native hyaline articular cartilage. In advanced osteoarthritis, none of the original hyaline cartilage may remain (Fig. 3-12). Although type II collagen continues to be produced36 in osteoarthritic repair cartilage, a class switch to type I collagen23 has been demonstrated in more advanced osteoarthritis37 as well as marked loss of type VI collagen from the perilanucunar matrix.38
The cartilaginous surface of osteophytes is covered by a mixture of fibrocartilage and fibrous tissue, at times overlying a residual area of hyaline cartilage and original subchondral bone (Fig. 3-13). For these reasons, biochemical and molecular studies of osteoarthritic cartilage frequently produce heterogeneous results, depending on the admixture of native cartilage, repair cartilage, and fibrocartilage in the specimens examined.
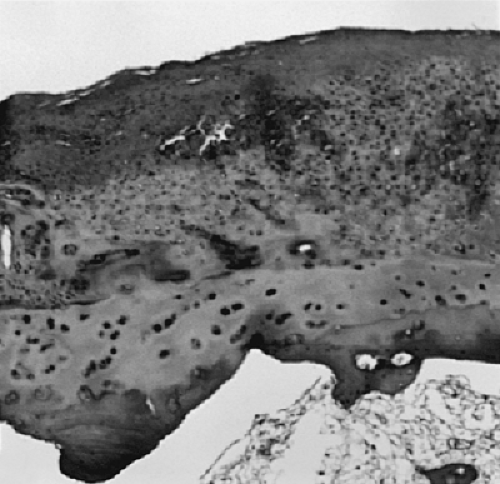 Figure 3-11 Cellular repair fibrocartilage on surface of joint. Note underlying residual hyaline articular cartilage. (Magnification x 50.) |
The tidemark, as seen in Fig. 3-1, is defined as an undulating hematoxyphilic line marking the boundary of the zone of calcified cartilage with the deep radial zone of articular cartilage. In osteoarthritis, this line becomes extensively reduplicated, often with irregular projections of calcified cartilage into the basal articular cartilage (Fig. 3-14). Several types of degenerative change in the zone of calcified cartilage have been reported39 in association with osteoarthritis.
Degenerative changes in the zone of calcified cartilage, including reduplication and advancement into the basal noncalcified articular cartilage, occur early in the course of osteoarthritis, often apparent in underlying areas with only minimal fibrillation. As a result, considerable speculation remains as to the possible role of changes in the calcified cartilage in initiating or promoting osteoarthritis.40,41 Advancement of the vascularized subchondral ossification front could contribute to thinning of the articular cartilage, thereby leading to progression of osteoarthritis. It may also be related to the re-expression of vascular endothelial growth factor by chondrocytes noted in an experimental model.42
Subchondral Bone in Osteoarthritis
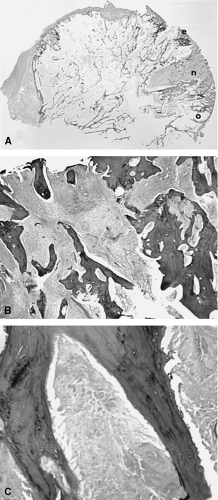
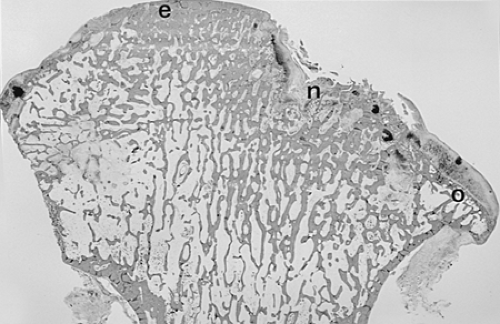
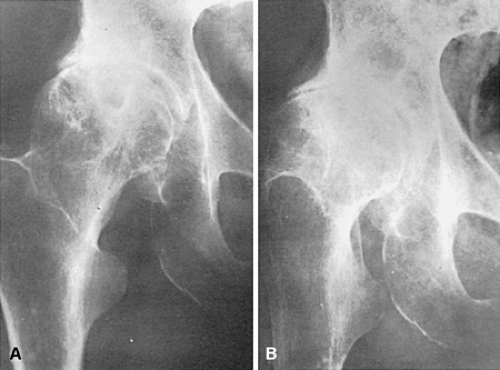
As heretofore discussed, remodeling of the bone-cartilage interface occurs early in the course of osteoarthritis, often in areas underlying fibrillated articular cartilage. Several other forms of remodeling also occur in the subchondral bone, either directly beneath the weight-bearing surface or at the margins of the joint. The latter constitute the osteophytes so characteristic of primary osteoarthritis. Proliferation of bone in the subchondral areas leads to remodeling of the bone-calcified cartilage interface with vascular incursion into the articular cartilage.8,43 Eventually, spikes of granulation and fibrous tissue reach the joint surface. Enchondral ossification, together with intramembranous ossification of fibrovascular tissue penetrating the cartilaginous surface (Fig. 3-15), produces thinning of cartilage44 and eventual exposure of smooth, dense bone on the articular surface (Fig. 3-16). This phenomenon, known as eburnation, is characterized not only by dense bone at the articular surface, but also by marked sclerosis of the subchondral cancellous bone. This change can be observed while some cartilage remains on the joint surface but is most marked when cartilage is totally absent as suggested by quantitative studies.45 At times, the resulting dense bone demonstrates secondary osteonecrosis characterized by small zones of devitalized bone with empty lacunae devoid of viable osteocytes. Although usually appreciated only on microscopic examination, the zones are large enough at times to be visible grossly (Fig. 3-17). The appearance is distinctly different from primary osteonecrosis (avascular necrosis), in which a subchondral bony sequestrum underlies viable articular cartilage. The
role of this secondary osteonecrosis in promoting collapse of the articular surface (Fig. 3-18) in advanced osteoarthritis has been the subject of discussion for years. The pathogenesis of this condition is presumably related to occlusion of minute intramedullary arteries. At any rate, it is common, occurring in up to14% of femoral heads resected for severe osteoarthritis.46 In addition, some patients with osteoarthritis develop sterile subchondral inflammatory microabscess-like accumulations.47 The pathogenesis of these zones is uncertain, but they may contribute to collapse associated with rapidly progressive variants of osteoarthritis.
role of this secondary osteonecrosis in promoting collapse of the articular surface (Fig. 3-18) in advanced osteoarthritis has been the subject of discussion for years. The pathogenesis of this condition is presumably related to occlusion of minute intramedullary arteries. At any rate, it is common, occurring in up to14% of femoral heads resected for severe osteoarthritis.46 In addition, some patients with osteoarthritis develop sterile subchondral inflammatory microabscess-like accumulations.47 The pathogenesis of these zones is uncertain, but they may contribute to collapse associated with rapidly progressive variants of osteoarthritis.
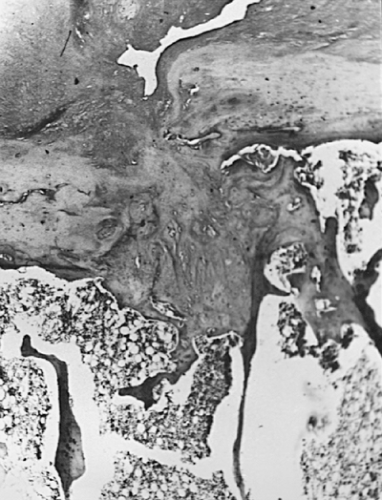
Microfractures of subchondral bone trabeculae also have a potential role in the provocation and progression of osteoarthritis. Subchondral trabecular fractures must be distinguished from microfractures of the calcified cartilage (Fig. 3-3) and bony plate.48 These allow vascularization of the cartilage, and when communicating with the surface, provoke intra-oseous pseudocyst formation (Fig. 3-19). Vascular invasion of the basal calcified cartilage may lead to ossification and resulting thinning of the cartilage, increasing shear stresses.49 Microfractures are easily demonstrated in load-bearing zones of subchondral bone,50 but they are decreased in osteoarthritis.49,51 This suggests the remodeling of bone into thicker, less compliant trabeculae52 may be the primary event in producing
cartilage damage rather than microfractures themselves. Remodeling of bone in osteoarthritis has been confirmed by both direct observation and fractal analysis.53
cartilage damage rather than microfractures themselves. Remodeling of bone in osteoarthritis has been confirmed by both direct observation and fractal analysis.53
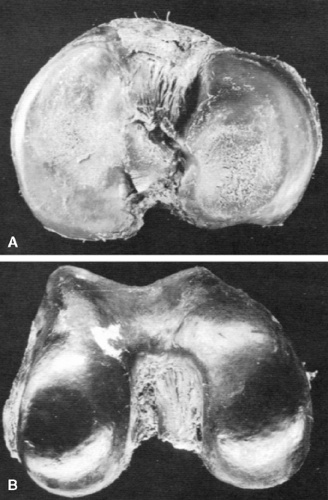
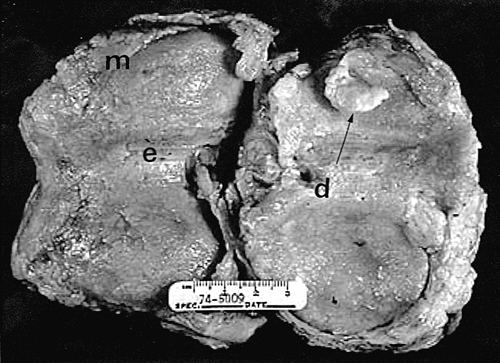
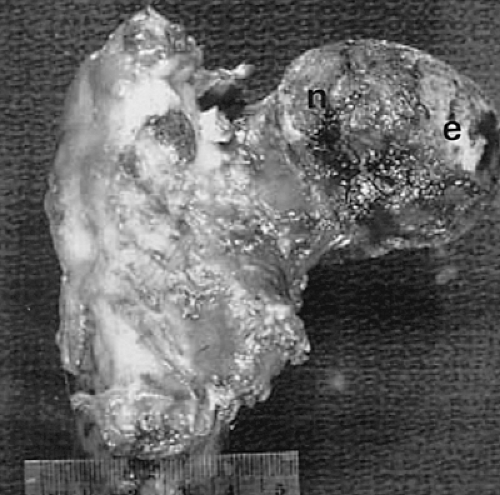
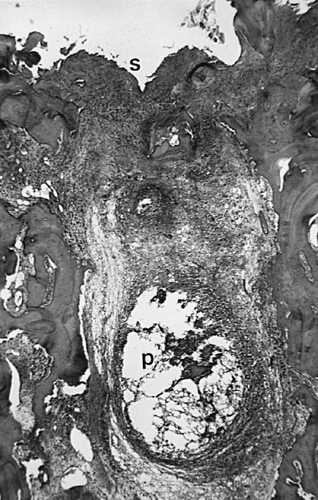
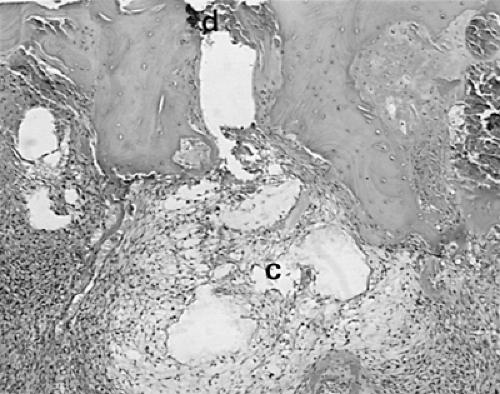
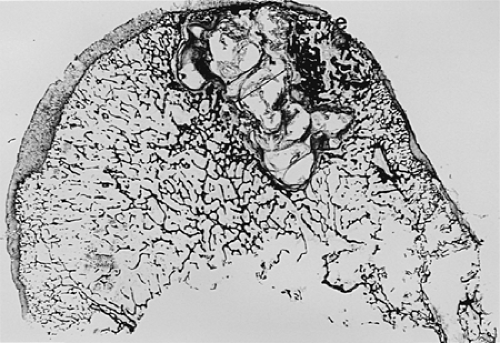
Another characteristic of progressive osteoarthritis is the formation of subchondral pseudocysts (Fig. 3-20). These are especially prominent in both the acetabular and femoral components of the hip joint. These spaces usually contain fluid and fibromyxoid material with occasional fragments of nonviable bone or cartilage. When mature, the pseudocyst is surrounded by a thin rim of reactive bone (Fig. 3-20). Minute gaps penetrating through the subchondral plate and articular cartilage are commonly seen at the apex of these pseudocysts, especially if serial sections are obtained (Fig. 3-19). The most attractive theory regarding pseudocysts is that the intrusion of intra-articular pressure through osteocartilaginous discontinuities produces local necrosis leading to the rarefied zones.54 This is also supported by the observation that the pseudocysts tend to disappear as osteoarthritis progresses to an advanced state with the joint surface being replaced by either a solid layer of eburnated bone (Fig. 3-16) or repair fibrocartilage (Fig. 3-21). Enchondral bone formation is characteristic of progressive osteoarthritis. This may take the form of enchondral bone formation at the base of articular cartilage (Fig. 3-13) or at the margins of the joint.55 The latter type, when fully developed, leads to osteophytes (Fig. 3-22). These bony outgrowths characteristically appear in areas away from the major weight-bearing zones. Large osteophytes are particularly characteristic of primary osteoarthritis of the hip joint; their absence suggests that osteoarthritis, if it is present, has resulted from prior inflammatory lysis of cartilage resulting in secondary rather than primary osteoarthritis (Fig. 3-23). Another characteristic phenomenon is the presence of osteochondral loose bodies known as joint mice. These fragments are composed of proliferative cartilage surrounding devitalized bone (Fig. 3-24). Their origin from the disordered joint surface is proved by the presence of corresponding defects left by their avulsion (Fig. 3-4). Whether the primary factor in their genesis is underlying subchondral necrosis, disordered enchondral ossification, or mechanical avulsion is difficult to prove. The primacy of cartilage versus bone alterations in the provocation of osteoarthritis remains debatable. However, there can be no doubt that the latter stages are characterized by marked proliferative and degenerative changes of subchondral and marginal bone.
Periarticular Soft Tissues in Osteoarthritis
Current theories focus on osteoarthritis as a disorder resulting from aberrant responses of articular cartilage and subchondral bone to cytokines produced both systemically and locally.56,57 Inflammatory aspects of osteoarthritis have, until recently, attracted less interest.58,59 This should not obscure the fact that some degree of synovial villous
hypertrophy accompanied by fibrosis (Fig. 3-25) is common in osteoarthritis.60 It is important to distinguish the stage of any particular case of osteoarthritis before ascribing causation to the synovial inflammation. This is one reason that autopsy hip joints with comparatively mild osteoarthritis do not reflect the synovial pathology of surgical specimens from hip replacement.2 The synovial response in osteoarthritis has been postulated to evolve from an early exudative stage characterized by intimal hyperplasia to a late fibrotic stage.60 Most studies of synovium in early osteoarthritis have shown that the synovitis is characterized histologically by a mild infiltrate composed primarily of lymphocytes and mononuclear cells61 and that the infiltrates differ both qualitatively and quantitatively from those associated with rheumatoid arthritis.62 Differences include greater overall cellularity61 and numbers of macrophages,63 plasma cells, and CD4 T cells64 in rheumatoid arthritis as opposed to osteoarthritis. Although the cellular and molecular character of the infiltrate in osteoarthritis clearly differs from that in rheumatoid arthritis, the presence of inflammation is undeniable. In one study, higher levels of the inflammatory marker C-reactive protein in serum of women predicted progression of early knee osteoarthritis.65 Although cells with CD4 phenotype are more plentiful in rheumatoid arthritis,64,66 cells with CD16+/56 phenotype indicative of natural killer cell activity are actually more numerous in osteoarthritis synovium.66 Mast cells, with possible roles in mediating inflammation and bone destruction, are increased in the synovium in osteoarthritis and in one study were significantly more numerous than in rheumatoid synovium.67 Other studies have examined the relationship of low-grade synovial inflammation in osteoarthritis to cytokine production.68 Chondrocytes responding to chronic cytokine stimulation may well produce the degradation of matrix macromolecules, including type II collagen, so characteristic of progressive osteoarthritis.34,35
hypertrophy accompanied by fibrosis (Fig. 3-25) is common in osteoarthritis.60 It is important to distinguish the stage of any particular case of osteoarthritis before ascribing causation to the synovial inflammation. This is one reason that autopsy hip joints with comparatively mild osteoarthritis do not reflect the synovial pathology of surgical specimens from hip replacement.2 The synovial response in osteoarthritis has been postulated to evolve from an early exudative stage characterized by intimal hyperplasia to a late fibrotic stage.60 Most studies of synovium in early osteoarthritis have shown that the synovitis is characterized histologically by a mild infiltrate composed primarily of lymphocytes and mononuclear cells61 and that the infiltrates differ both qualitatively and quantitatively from those associated with rheumatoid arthritis.62 Differences include greater overall cellularity61 and numbers of macrophages,63 plasma cells, and CD4 T cells64 in rheumatoid arthritis as opposed to osteoarthritis. Although the cellular and molecular character of the infiltrate in osteoarthritis clearly differs from that in rheumatoid arthritis, the presence of inflammation is undeniable. In one study, higher levels of the inflammatory marker C-reactive protein in serum of women predicted progression of early knee osteoarthritis.65 Although cells with CD4 phenotype are more plentiful in rheumatoid arthritis,64,66 cells with CD16+/56 phenotype indicative of natural killer cell activity are actually more numerous in osteoarthritis synovium.66 Mast cells, with possible roles in mediating inflammation and bone destruction, are increased in the synovium in osteoarthritis and in one study were significantly more numerous than in rheumatoid synovium.67 Other studies have examined the relationship of low-grade synovial inflammation in osteoarthritis to cytokine production.68 Chondrocytes responding to chronic cytokine stimulation may well produce the degradation of matrix macromolecules, including type II collagen, so characteristic of progressive osteoarthritis.34,35
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree