Pathogenesis
Pauline M. Camacho
Bone Remodeling Cycle
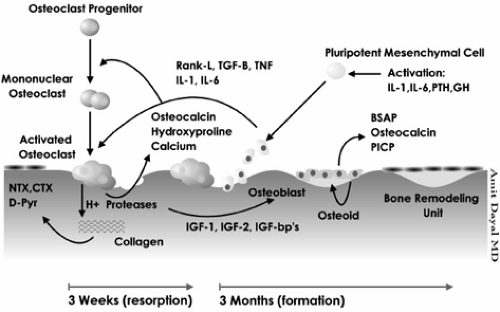
Bone is in a constant state of turnover or activity throughout life. Remodeling is the process of renewing bone by breaking down old bone and forming new bone. At the very core of this process is a unit, or group of cells, called the bone-remodeling unit (BRU). There are four distinct stages of bone remodeling, specifically, activation, resorption, reversal, and formation (Fig. 2.1).
Activation is initiated by the recruitment of osteoclast precursors into the areas that need to be resorbed. It is not yet exactly clear how the body determines which areas need to be remodeled. The precursor (mononucleated) cells fuse to become preosteoclast (multinucleated) cells and mature further into osteoclasts. The factors that are thought to regulate the process of osteoclast maturity include local factors such as receptor activator of nuclear factor-κB ligand (RANKL), interleukin-1 and -6 (IL-1, IL-6), colony-stimulating factors (CSF-1), tumor necrosis factor (TNF), transforming growth factor βb (TGF-βb), and systemic factors such as parathyroid hormone (PTH), 1,25-hydroxyvitamin D (OHD), and calcitonin [1,2,3,4,5]. Once activated, the osteoclasts acidify and release resorptive enzymes, leading to the formation of resorption cavities. After their job is finished, the osteoclasts undergo apoptosis.
This process is followed by the reversal stage, during which coupling signals are sent to attract osteoblasts into the resorptive sites. Resorption is then turned off, and the formation stage follows. The osteoblasts synthesize bone matrix and facilitate its mineralization. Calcium and phosphate ions are deposited into the matrix, leading to hardening of the bone. Osteoblasts undergo apoptosis, become encased within the mineralized matrix to become osteocytes, or evolve into bone-lining cells. The osteocytes maintain communication with each other, and they likely play a role in sensing the areas that need to be remodeled, transmitting information to other cells, and initiating the bone-remodeling process. The end product is the formation of new bone; this is where a negative balance may occur, leading to osteoporosis.
Postmenopausal Osteoporosis
The basic pathology in osteoporosis is an imbalance between bone resorption and bone formation. The most common cause of this imbalance is menopause. The bone mass of an elderly woman is the product of her peak bone mass and bone loss that she has incurred for various reasons (Table 2.1).
Table 2.1. Factors that play a role in osteoporotic bone loss | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
Peak Bone Mass
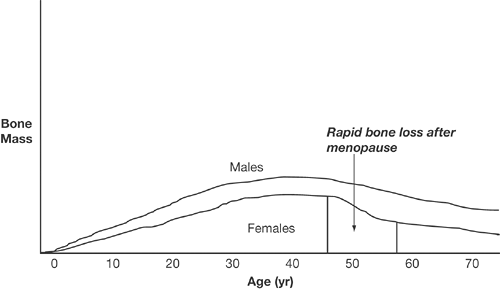
Puberty is characterized by rapid gain in bone brought on by surges in sex hormone levels. This gain continues until the peak is reached, at age 20 to 30 years (Fig. 2.2). Approximately 50% to 80% of a woman’s peak bone mass is determined by her genetic predisposition [6], but other factors such as calcium, vitamin D intake, activity level, low body weight, illnesses, or delayed puberty also contribute to the overall product.
Period of Bone Loss
After peak bone mass is reached, the bone remodeling process is in a state of equilibrium until menopause. Cessation of estrogen production leads to rapid bone loss of approximately 2% to 3% per year in the spine for up to 6 to 8 years, which accounts for 50% of the total spinal bone loss among normal women [7]. This is then followed by a slower rate of bone loss (0.5%/year), which is attributed to aging (Fig. 2.2) [7].
Why does estrogen deficiency lead to acceleration of bone loss? Estrogen deficiency causes an increase in activation frequency, perhaps due to local growth factors and cytokines, which affect osteoblastic and osteoclastic action, with the net effect being a negative bone balance at the end of each remodeling cycle. This is further supported by the fact that after menopause, markers of both bone resorption and formation increase, and a greater increase is seen in bone resorption markers.
Even among men, it is now known that estrogen deficiency plays a big role in bone loss, perhaps an even bigger role than played by testosterone [8]. Studies among osteoporotic males have shown a closer correlation between estradiol levels and bone mineral density (BMD) than testosterone and BMD. A finding that men with osteoporosis may have low estradiol yet normal testosterone levels further supported this correlation [9].
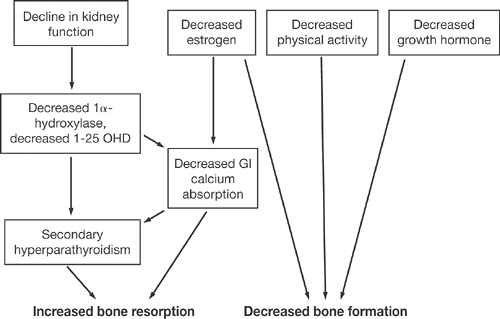
Age-related bone loss is attributable to various factors (Fig. 2.3). Decline in kidney function leads to decreased 1α hydroxylation of vitamin D, decrease in gut calcium absorption, secondary hyperparathyroidism, and subsequent increase in bone resorption. Estrogen deficiency also contributes to diminished calcium absorption from the gastrointestinal tract and secondary hyperparathyroidism. Other factors that occur with aging include decrease in physical activity and in growth hormone secretion, ultimately leading to diminished osteoblastic function [7].
Male Osteoporosis
Most men develop osteoporosis because of secondary causes of bone loss (which will be discussed in more detail in Chapter 6). Like bone gain in women, bone gain in men accelerates during adolescence, but the peak bone mass is reached a little later in life. Contrary to the situation in women, however, there is no period of dramatic and accelerated bone loss; rather, there is a steady decline, similar to what occurs after the menopausal years. In general, clear differences in the bone structure of men and women protect men from osteoporosis. Specifically, their bones are larger and the cortices thicker.
The age-related changes in women described previously also occur in men. Bone loss is similarly more pronounced in trabecular bone. Slight cortical thinning in the long bones does occur but does not appear to contribute much to an increased risk of fracture, as there is an increase in periosteal bone expansion that preserves bone strength [10].
Hypogonadism, whether idiopathic or induced by medications, is an increasing cause of osteoporosis among men. The use of androgen deprivation therapy has been clearly associated with bone loss among men with prostate cancer [11,12,13,14,15,16]. Bone loss occurs at a rate of 3% to 4% per year and can continue for up to 10 years of therapy [13].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree